Abstract
242326
Introduction: Cardiovascular dyssynchrony, prevalent in heart failure (HF), necessitates interventions such as Cardiac Resynchronization Therapy (CRT) to define the categories of symptomatic HF. Despite clear inclusion criteria defined by the guidelines, still, ~30%-40% of cases do not respond to CRT, highlighting the need to investigate novel markers. Various studies have explored indicators for CRT patient selection, including U-shaped contraction pattern, Left Ventricle (LV) dyssynchrony, scar tissue burden, and lead placement. Gated SPECT Myocardial Perfusion Imaging (GSPECT-MPI) can be considered a one-stop-shop modality as it can provide all novel and conventional metrics. Furthermore, recent studies in quantitative radiomics analysis have shown potential as biomarkers in coronary artery disease (CAD), for diagnosis, prognosis, and survival analysis. We aimed to assess CRT response using quantitative features including conventional quantitative features and radiomic features extracted from GSPECT MPI images before CRT treatment using machine learning (ML) algorithms.
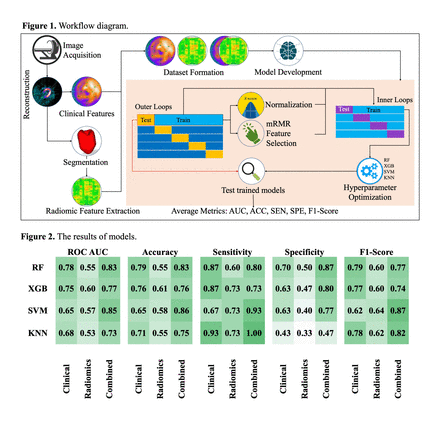
Methods: In this retrospective study, data were collected from 28 patients (mean age = 73.7 ± 8.9) who underwent CRT treatment and GSPECT MPI before treatment. The flowchart of this study is given in Figure 1. Image acquisition of rest GSPECT MPI involved dual-head gamma camera imaging (Symbia™ T2, Siemens Healthcare), followed by Ordered Subset Expectation Maximization (OSEM) reconstruction (4 iterations; 4 subsets) and Butterworth (cut-off=0.40, order=5) post-filter. Phase analysis and polar map assessment were conducted using Cedars-Sinai CSI software to collect clinical features (Tables 1 and 2). LV segmentation and radiomics feature extraction were performed using ITK-SNAP software and Pyradiomics, respectively, yielding 107 features in different families including shape, intensity, and second-/high-order texture features from GLDM, GLCM, GLRLM, GLSZM, and NGTDM. ML analysis was carried out on three datasets (clinical, radiomics, and combined) using Random Forest (RF), eXtreme Gradient Booting (XGB), Support Vector Machine (SVM), and K-Nearest Neighbor (KNN) algorithms along with minimum Redundancy - Maximum Relevance (mRMR) feature selection method (number of selected features in each model=7) and nested cross-validation (outer loop=5, inner loop=4), with evaluation metrics including AUC, accuracy (ACC), sensitivity (SEN), specificity (SPE), and F1 Score.
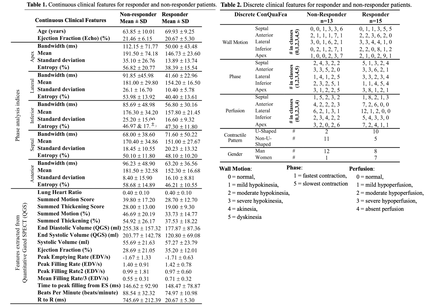
Results: The performance of the models can be seen in Figure 2. The best model for clinical features was RF (AUC, ACC, SEN, SPE, and F1 Score as 0.78 ± 0.16, 0.79 ± 0.16, 0.87 ± 0.26, 0.70 ± 0.16, 0.79 ± 0.20), for radiomics was XGB (AUC, ACC, SEN, SPE, and F1 Score as 0.60 ± 0.14, 0.61 ± 0.15, 0.73 ± 0.39, 0.47 ± 0.12, 0.60 ± 0.31), and for combined was SVM (AUC, ACC, SEN, SPE, and F1 Score as 0.85 ± 0.08, 0.86 ± 0.07, 0.93 ± 0.13, 0.77 ± 0.20, 0.87 ± 0.07). The majority of metrics improved after using the datasets combined.
Conclusions: Our study aimed to assess the response to CRT treatment by incorporating quantitative features, including clinical and radiomic features, using ML techniques. The findings suggest that both datasets especially radiomic features alone are insufficient to evaluate treatment response adequately. However, their combination leads to enhanced predictive power of the models with even lower standard deviation for different metrics. Utilization of the mRMR feature selection method, selecting the most informative feature and those with the potential to boost its predictive power, contributed to this outcome, enabling synergy among features in these datasets. It can be concluded from the promising results of this study that using newly introduced features combined with radiomic features, and employing machine learning algorithms, provides the potential to enhance the accuracy in selecting patients for CRT treatment.