Abstract
242286
Introduction: Theranostic radiometal-based radiopharmaceuticals (RPs) attract a great deal of interest as they have the potential to non-invasively image (via diagnostic scans) and treat cancer (via therapeutic radiation) using the same drug construct, allowing for personalized treatment options. Mercury-197m/g (197m/gHg, t1/2 = 23.8 h/ 64.14 h) forms a matched theranostic-pair, which can be used as a diagnostic agent via single-photon emission computed tomography (SPECT) imaging and as a therapeutic one by harnessing concurrent emissions of γ-rays and cytocidal Auger electrons, respectively.
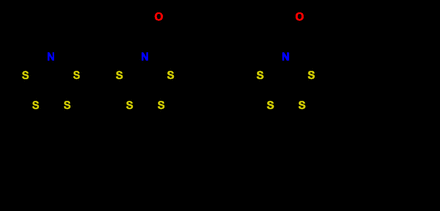
A vital component of an inorganic RP is the bifunctional chelator (BFC), which joins the radiometal to a tumor-seeking vector for site-specific radiation delivery to diseased cells. However, the lack of suitable chelating agents capable of forming thermodynamically stable and kinetically inert complexes with 197m/gHg radioisotopes in vivo hampers potential applications. Research efforts are focused on developing 197m/gHg tailored BFCs. Our previous work identified two macrocyclic NS4 ligands (NS4 and NS4-BA) with results suitable for incorporation into a 197m/gHg radiopharmaceutical. As a result, the BFC derivative of the NS4 ligand, functionalized with a tetrazine moiety that can undergo a selective biorthogonal "click" reaction in vivo, enabling pre-targeted studies, was synthesized and studied herein.
Methods: The NS4 ligands and their non-radioactive Hg2+ complexes have been synthesized (Fig. 1) and characterized using conventional spectroscopic techniques. Radiolabeling of the NS4 ligands with 197m/gHg and in vitro stability of the resulting complexes were studied and analyzed via radio-TLC and SDS-PAGE, respectively. The BFC derivative (NS4-Tz, Fig. 1) has also been synthesized, characterized, and "clicked" to the monoclonal antibody, trastuzumab. MALDI-MS was used to determine the number of chelators attached per antibody. Radiolabeling yields of the BFC and the bioconjugate with 197m/gHg have been explored along with in vitro stability using previously mentioned methods. This work will lead to further in vitro cell and in vivo studies
Results: The ligands were synthesized, and non-radioactive Hg2+ complexes formed rapidly at millimolar concentrations and produced highly stable 1:1 metal-to-ligand complexes. NS4 and NS4-BA radiolabeled 197m/gHg after 1 h (pH 7) quantitatively (>90% RCY) at 80 °C ([L] = 10-5 M), and 37 °C ([L] = 10-4 M). 197m/gHg NS4-BA complexes remained 74% and 93% intact in human serum over 24 h and glutathione over 3 d. The BFC, NS4-Tz, was successfully synthesized and radiolabeled with 197m/gHg to ensure the ligand retained its binding affinity to 197m/gHg with the introduction of a tetrazine. NS4-Tz was successfully radiolabelled with 197m/gHg after 1 h (pH 7) quantitatively (>90% RCY) at 37 °C ([L] = 10-4 M). The complex kinetic inertness was studied against human serum and glutathione, resulting in 72% and 95% of the complex remaining intact over 24 h. Following this, in vivo [197m/gHg][Hg(NS4-Tz)]2+ complex stability studies were undertaken in healthy mice. SPECT images and biodistribution data concluded that the complexes remained intact in vivo over 4 h. Further, the [197m/gHg][Hg(NS4-Tz)]2+ labeled complex was conjugated to trastuzumab utilizing a 2-step labeling method to avoid non-specific binding with the antibody. MALDI-MS results showed 2.1 chelates per antibody. Future, targeted animal and cell studies using the SKOV-3 cancer cell line will be done to determine the efficacy of the radiopharmaceutical.
Conclusions: Novel NS4 ligands are optimal for chelation for the theranostic radioisotopes 197m/gHg, and have been incorporated into a BFC and bioconjugate. Ongoing studies will evaluate radiomercury's targeted imaging and therapeutic abilities, which have been limited in the literature thus far. Developing a library of 197m/gHg RPs is highly impactful in accessing the untapped potential of radioisotopes in clinical applications.