Abstract
242272
Introduction: PET imaging studies are critical to our understanding of the endocannabinoid system (ECS), which is involved in several neuropsychiatric illnesses and addictions. The ECS consists of two main cannabinoid receptors, CB1 and CB2, endocannabinoids 2-arachidonoylglycerol (2-AG) and anandamide, as well as major synthesis/metabolism enzymes such as diacylglycerol lipase (DAGL) and monoacylglycerol lipase (MAGL). The first aim of this project is to develop automated syntheses and validate [18F]FMPEP-d2 (CB1) (Donohue, J. Med. Chem., 2008) and [18F]MAGL-2102 (Rong, J. Med. Chem., 2021) for human use. Secondly, we will explore the application of both radiotracers to study the ECS in mouse models of Alzheimer’s disease (AD) and psychosis.
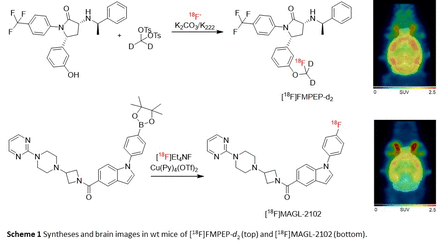
Methods: The radiosynthesis of [18F]FMPEP-d2 was automated using a commercial synthesis module (GE Tracerlab FXN) via a novel one-pot SN2 reaction of [18F]fluoride with ditosylmethane-d2 and PPEP (Scheme 1; Pees & Vasdev, J. Fluorine Chem, 2023). The copper-mediated [18F]MAGL-2102 synthesis from the boronic ester precursor was also automated (Scheme 1). PET-CT images with both tracers in female and male AppNL-G-F (AD amyloid-ß (Aß) model) and dopamine transporter knock-out mice (DAT-KO; psychosis model) as well as non-transgenic wild-type mice (wt) were dynamically acquired over 120 min. Image analyses and extraction of brain TACs from the dynamic images were performed using PMOD with standard MR brain templates and atlases for mice. After the PET scans, the brains were collected for further analysis.
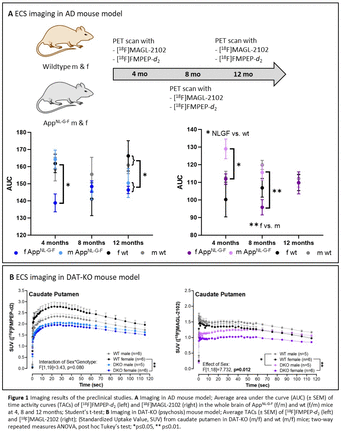
Results: [18F]FMPEP-d2 was obtained with a radiochemical yield (RCY) of 8±1% (decay-corrected, n=8), a molar activity (Am) of 322±101 GBq/µmol and radiochemical purity (RCP) >95% within 70 minutes. [18F]MAGL-2102 was obtained with a RCY of 14±4% (dc, n=4), Am of 506±302 GBq/µmol and RCP >95%. PET imaging of the tracers in the AD mouse model showed time- and sex-dependent changes in CB1 and MAGL availability (Figure 1A). While at early stages of Aß pathology (4 months) female AppNL-G-F mice had a lower CB1 availability across the whole brain, MAGL availability was increased in AppNL-G-F mice compared to wt mice of both sexes. At intermediate stages (8 months), no differences in CB1 or MAGL availability were observed between genotypes, but MAGL availability strongly differed between sexes (m>f). At 12 months (robust Aß pathology), a significantly lower uptake of [18F]FMPEP-d2 was observed in AppNL-G-F compared to wt mice, but no changes in [18F]MAGL-2102 binding were observed. Evaluation of the tracers in the psychosis model (>4 months) showed a decrease in [18F]FMPEP-d2 binding in female DAT-KO mice compared to female wt mice in all brain regions, whereas the decrease in male mice was not significant. [18F]MAGL-2102 binding was decreased across all brain regions in DAT-KO mice of both sexes compared to the wt mice, with males generally having higher MAGL availability than females (Figure 1B).
Conclusions: Automated syntheses of [18F]FMPEP-d2 (CB1) and [18F]MAGL-2102 were achieved and human studies are planned at our laboratories. PET imaging in the AD mouse model showed increased MAGL availability at early stages of Aß pathology and reduced CB1 availability at later stages, suggesting that MAGL might be a useful target for early detection and therapy. PET imaging in a psychosis model showed that both MAGL and CB1 availability is decreased in chronic high dopamine states, indicating that decreasing 2-AG by inhibition of DAGL could be therapeutic. Both tracers show sex differences in the ECS which should be considered when developing ECS-targeted diagnostics and therapies.