Abstract
242266
Introduction: Triple-negative breast cancer (TNBC) represents a highly aggressive subtype with a poor prognosis, and targeted therapies such as endocrine or anti-HER-2 are lacking. Heterogeneous responses to standard chemotherapeutic regimes further complicate the clinical management of TNBC and highlight an unmet need for better therapeutics. We have developed several alkyphosphocholine analogs (NM600) as tumor-selective targeted radioligands for radiopharmaceutical therapy (RPT) of breast cancer. Herein, we investigate 225Ac-NM600 therapeutic efficacy and safety in an aggressive metastatic mouse model of TNBC.
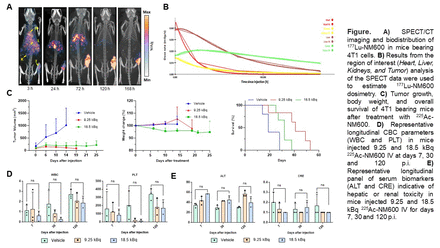
Methods: Female Balb/c 10-12 weeks old were subcutaneously implanted 4T1 cells (5 × 105 cells) on the right flank, and tumor volume was determined via caliper measurement using the ellipsoid volume formula. In vivo imaging or TRT studies were conducted when the tumor reached a volume of ~350 mm3. NM600 was radiolabeled with 177Lu as a SPECT/CT imaging surrogate of therapeutic 225Ac-NM600. In imaging studies, 4T1 bearing mice (n = 4) were injected with 13 MBq of 177Lu-NM600 intravenously, and sequential scans were acquired in MI Labs SPECT/CT at 3, 24, 72, 120 and 168 h post injection (p.i.) of the radiotracer. Region-of-interest (ROI) analysis of the SPECT images was performed to determine the magnitude and kinetics of 177Lu-NM600 uptake in the tumor and normal tissues of interest. Results from the ROI analysis of the longitudinal SPECT data were used to estimate 177Lu-NM600 dosimetry using the Imalytics software. RPT studies were conducted in randomly selected mice bearing 4T1 tumors. Three groups of mice (n = 5) received a single 9.25, or 18.5 kBq IV injection of 225Ac-NM600. Tumor volume and overall survival were monitored thrice weekly for 55 days. 225Ac-NM600 toxicity was evaluated in groups of Balb/c naïve mice (n = 3) administered 9.25 or 18.5 kBq of 225Ac-NM600 or vehicle at days 7, 30, and 120 p.i. through complete blood count (CBC), blood chemistry, and histopathological analysis.
Results: SPECT imaging and biodistribution studies demonstrated in vivo selective tumor uptake and retention of 177Lu-NM600. Tumors exhibited increasing 177Lu-NM600 accumulation (8.31 ± 2.65 %IA/g at 3 h p.i.; n = 4) reaching peaked values of 15.2 ± 3.3 %IA/g at 120 h p.i. due to prolonged radioactivity retention. In contrast, 177Lu-NM600 accumulation in the liver, which reached peak uptake values of 13.73 ± 1.64 %IA/g at 3 h p.i., gradually declined, indicating hepatobiliary excretion of the tracer. Tumor and normal organs dosimetry for 177Lu-NM600, estimated from SPECT/CT imaging, showed that the tumor received the highest integral absorbed dose per activity of all tissues. Among normal tissues, the liver demonstrated comparably elevated absorbed doses. Mice bearing 4T1 tumors treated with 225Ac-NM600 exhibited significant tumor growth inhibition (p ≤ 0.015), leading to an extended survival rate. Median survival of 18, 30, and 42 days was recorded for the vehicle, 18.5 kBq and 9.25 kBq groups, respectively. Interestingly, despite effectively controlling the primary tumor, the highest IA led to faster metastatic progression and death. Mice administered 225Ac-NM600 9.25 or 18.5 kBq exhibited transient bone marrow toxicity characterized by reductions in white blood cells, lymphocytes, red blood cells, and hemoglobin levels at 10 and 30 days p.i. Notably, the observed effects were mild, even at the highest activity level, and did not lead to animal mortality.
Conclusions: These findings underscore the potential of 225Ac-NM600 as a promising new therapeutic avenue for TNBC management, warranting further assessments of efficacy and potential toxicities in additional TNBC animal models. Furthermore, our approach opens the door for novel, more effective therapeutic combinations leveraging the genotoxic and immunomodulatory properties of 225Ac RPT agents.