Abstract
242152
Introduction: The antibody-drug conjugate (ADC) PADCEV (enfortumab vedotin, Astellas) targets nectin-4 and delivers the microtubule-disrupting agent monomethyl auristatin E (MMAE) to tumors. PADCEV has recently been approved for the treatment of primary and metastatic urothelial bladder cancer, and is actively being explored clinically across a wide range of other solid malignancies. Identifying the patients most likely to respond to PADCEV is crucial given the heterogeneous nature of nectin-4 expression, side effect profile and high cost of the ADC. In this work, we adapted PADCEV into an immunoPET agent as a means to non-invasively assess nectin-4 expression.
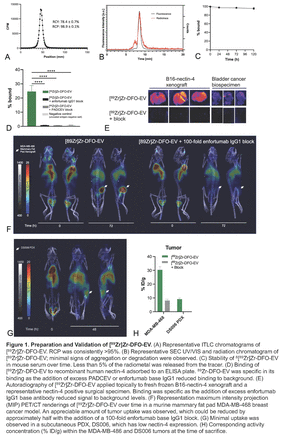
Methods: The radiotracer termed [89Zr]Zr-DFO-EV was prepared by randomly conjugating deferoxamine (DFO) to amines on PADCEV, followed by coordination with [89Zr]Zr-oxalate under slightly acidic conditions in a phosphate-based buffer. Unbound radiometal was removed using centrifugal micro-concentration and successive washes with 1x PBS. Radiochemical purity (RCP) and serum stability were assessed using instant thin-layer chromatography (ITLC). Size exclusion chromatography (SEC) was used to assess the conformation of the radiotracer, and a plate-binding assay was used to ensure that [89Zr]Zr-DFO-EV could still bind selectively to its target antigen. Binding assays were also performed against nectin-4 positive cells and ex vivo surgical biospecimens of urothelial carcinoma using autoradiography (MSKCC biospecimen protocol 18-148). Next, serial PET/CT images over a 48-72 h period were acquired following intravenous administration of [89Zr]Zr-DFO-EV in mice bearing subcutaneous and/or orthotopic xenografts across a range of cancer types (skin, bladder, breast, HNSCC) and nectin-4 expression. PET/CT imaging was also carried out in mice bearing patient derived xenografts of urothelial carcinoma origin with low and high nectin-4 expression.
Results: The RCP of [89Zr]Zr-DFO-EV was 98.9 ± 0.1% and the radiochemical yield (RCY) was roughly 80% [Figure 1A]. Greater than 95% of the radiometal remained bound to the radiotracer when challenged in serum over a 5 d period [Figure 1B]. Minimal signs of degradation or aggregation were observed in SEC analysis of [89Zr]Zr-DFO-EV, which co-eluted at the same time as unmodified PADCEV [Figure 1C]. [89Zr]Zr-DFO-EV bound to recombinant human nectin-4 in a plate binding assay, which was blockable with excess unmodified PADCEV or the enfortumab base IgG1 [Figure 1D]. Specific and blockable binding was also observed for topical staining of B16 cells overexpressing nectin-4 and nectin-4 positive biospecimens [Figure 1E]. In vivo, [89Zr]Zr-DFO-EV accumulated well (~20-30% ID/g) into nectin-4 positive xenografts and PDX models; the addition of 100-fold excess enfortumab base IgG1 block reduced uptake to that of nectin-4 negative lesions (~5-10% ID/g). Representative PET/CT MIP renderings [Figure 1F, G] and terminal biodistribution data [Figure 1H] for a high-expressing orthotopic breast model (MDA-MB-468) and a low-expressing nectin-4 PDX (DS006).
Conclusions: PADCEV was successful adapted into an immunoPET agent and validated across a wide-range of xenografts, PDXs and clinical biospecimens. Efforts are currently underway to translate this immunopET agent clinically to help identify patients most likely to benefit from PADCEV-based therapy.