Abstract
242100
Introduction: 177Lu-PSMA-617 is a radioligand therapy (RLT) indicated for the treatment of metastatic castration-resistant prostate cancer (mCRPC) patients who experienced biochemical recurrence after receiving androgen receptor pathway inhibition therapy and taxane-based chemotherapy. 177Lu-PSMA-617 is prescribed for six cycles of 7.4 GBq (200mCi) every six weeks. PSA level is used as a marker for therapy response. Treatment outcomes are diverse and difficult to predict. Our study aims to investigate factors affecting treatment failure or success in patients with mCRPC undergoing therapy with 177Lu-PSMA-617.
Methods: Patients with mCRPC who completed or failed 177Lu-PSMA-617 treatment at our institution from January 2022 to October 2023 were retrospectively evaluated. We divided our population into two groups; group one (treatment success group) included those who received six cycles of 177Lu-PSMA-617 and demonstrated biochemical response, and group two (treatment failure group) included patients who discontinued their treatment before cycle six due to biochemical failure. Patients who demonstrated poor performance and enrolled in hospice were excluded from this analysis. Data collection was performed for each patient utilizing REDCap database (Research Electronic Data Capture hosted at the University of Arizona, Tucson, AZ, USA). PSMA PET/CT scans were evaluated using MIM imaging software (Beachwood, Ohio). Statistical t-test analysis was performed. A p-value of <0.05 was considered statistically significant.
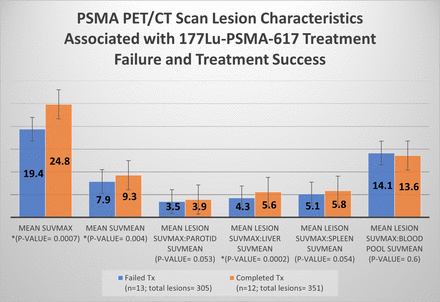
Results: A total of 119 cycles of 177Lu-PSMA-617 therapy were administered to a cohort of 25 patients. Among the 12/25 (48%) patients that completed six cycles with a biochemical response, the mean age (±SD) was 77 (±8.9), and patients had a total of 351 (29, ±7.8) lesions (average, ± SD) on pre-therapy PSMA PET/CT scan. The mean decrease of PSA was -72.9 ng/ml compared to the baseline. Among the 13/25 (52%) patients who experienced biochemical failure and discontinued therapy prior to the completion of 6 cycles, the mean age was 78 (±9.2), with a total of 305 (23, ±26.4) lesions on pre-therapy PSMA PET/CT scan. The mean PSA was 32.8 ng/ml higher than baseline. In this group, patients received an average of 3.6 (±1.1) cycles of 177Lu-PSMA-617. Lesions mean of SUVmax, SUV mean, and lesions SUVmax to liver SUVmean ratios were significantly higher in patients with the biochemical response; Success group vs. failure group had mean SUVmax of 24.8 vs. 19.4 (p=0.0007), SUV mean of 9.3 vs. 7.9 (p=0.004), lesion SUVmax to Liver SUVmean ratio of 5.6 vs. 4.3 (p=0.0002), respectively. Factors that were not associated with therapy failure or success (p>0.05) included lesions SUVmax to SUVmean ratios of the parotid, spleen, and blood pool.
Conclusions: The use of SUVmax, SUVmean, and ratio of tumor SUVmax to SUVmean ratio of liver may potentially be used as predictors for treatment response. Further evaluation of cut-off values with region under the curve (ROC) analysis can be obtained. Yet, SUV values are scanner/reconstruction dependent and, therefore, should be standardized accordingly.