Abstract
241873
Introduction: Heterodimer LNC1007 (NOTA-FAPI-RGD) that targets both fibroblast activation protein (FAP) and integrin αvβ3 has been developed and labeled with 68Ga and 18F for PET imaging of FAP positive and/or integrin αvβ3 positive tumors. In this study, we synthesized dual targeting LNC1009 (DOTA-EB-FAPI-RGD) that is derived from LNC1007 but with albumin binder Evans blue (EB) moiety and radiolabeled with 177Lu for cancer imaging and therapy.
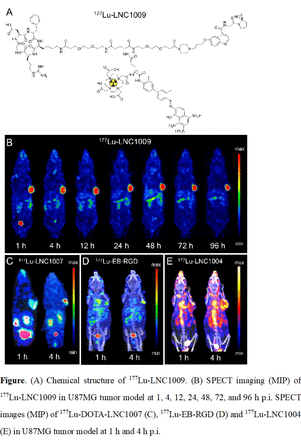
Methods: LNC1009 was designed and synthesized based on EB moiety, FAPI-02 inhibitor and cyclic RGDfK peptide. Binding affinity and targeting specificity were verified through cell uptake and competition binding assay. SPECT imaging and preclinical pharmacokinetics of 177Lu-LNC1009 were determined in U87MG xenograft model to identify the treatment potential. For the comparison, SPECT images of 177Lu-DOTA-NC1007, 177Lu-EB-RGD, and 177Lu-LNC1004 (177Lu-EB-FAPI) were also performed in U87MG tumor model.
Results: The 177Lu-LNC1009 had good stability in saline for at least 96 h, and showed favorable binding affinity and specificity in vitro and in vivo. SPECT imaging of 177Lu-LNC1009 showed significantly improved tumor uptake and retention than those of 177Lu-DOTA-FAPI-RGD. In addition, 177Lu-LNC1009 also exhibited higher tumor-to-background ratios and lower normal organs uptake than 177Lu-LNC1004 and 177Lu-EB-RGD, which indicated the enormous potential for cancer therapy.
Conclusions: 177Lu-LNC1009 was successfully synthesized and radiolabeled with high radiochemical purity and stability. With significantly improved absolute tumor uptake and decreased normal organ uptake, 177Lu-LNC1009 has the potential to enhance therapy effect that promises clinical translation for the treatment of many types of solid tumors that are FAP+/αvβ3-, FAP-/αvβ3+, or FAP+/αvβ3+.