Abstract
241809
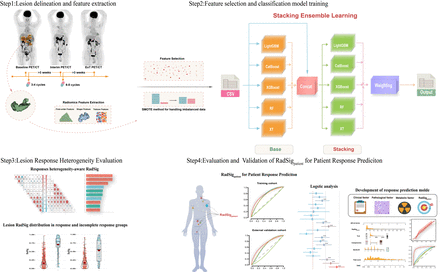
Introduction: Our aim is to develop and validate PET radiomics signatures (RadSig) using automatic machine learning (AutoML) for predicting treatment response of diffuse large B-cell lymphoma (DLBCL) patients.
Methods: A total of 308 DLBCL patients treated with R-CHOP-like regimen treatment from two independent medical centers with 1471 lesions were studied. AutoGluon, one of AutoML models, was applied to the baseline PET radiomic features from the training cohort to generate responses heterogeneity-aware RadSigs. The performance of RadSigs for predicting treatment response were validated in external cohort. Furthermore, multi-parametric prediction models were designed, and assessed through calibration curves, concordance index (C-index), and decision curve analysis (DCA).
Results: AutoML-generated responses heterogeneity-aware RadSigs were significantly higher in imcomplete response than that of response group in both training and external validation cohorts (P<0.05). The RadSigpatient for response prediction outperformed metabolic parameters (SUVmax, MTV and TLG) in training (AUC:0.834 vs 0.591 vs 0.718 vs 0.728 for interim; AUC: 0.809 vs 0.580 vs 0.739 vs 0.731 for EoT ) and external validation cohorts (AUC: 0.745 vs 0.512 vs 0.731 vs 0.722 for interim 0.707 vs 0.555 vs 0.652 vs 0.660 for EoT). The multi-parametric prediction models that incorporated RadSigpatient demonstrated superior efficacy and offered more net clinical benefits compared to competing models.
Conclusions: RadSigpatient generated by AutoML represent valuable biomarker for predicting treatment response in DLBCL patients, offering potential assistance in clinical decision-making.