Abstract
241756
Introduction: Liver cancer stands out as one of the most common malignancies worldwide. Yttrium-90 microsphere radioembolization shows promise as an innovative treatment method for liver cancers by selectively targeting arterial vessels that supply the tumor. This approach delivers radiation agents for effective tumor cell destruction. However, the efficacy of this method is hampered by extensive particle distribution in non-target areas. As such, precisely delivering the microspheres to target vessel outlets is crucial in optimizing treatment efficacy. In this study, we developed a 3D computational model to simulate transient particle hemodynamics in a representative human hepatic artery system.
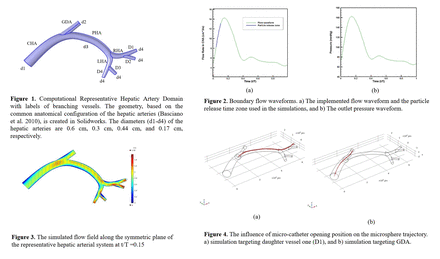
Methods: In this study, Eulerian fluid dynamics and Lagrangian particle transport are modeled by a one-way coupled system of partial differential equations. Blood was considered as an incompressible non-Newtonian fluid. Figure 1 illustrates the representative hepatic artery domain. Conservation equations of mass and momentum, along with boundary conditions of arterial flow rate (Figure 2a) and pressure (Figure 2b), derived from population-averaged data from Basciano et al. 2010, are utilized to simulate the microsphere transport. Calculated hemodynamics and spatial domain boundaries are used to calculate the forces acting on each particle. Subsequently, using Newton’s second law of motion for each particle, the trajectory of the yttrium-90 microsphere is obtained. Forces exerted on suspended particles include drag, pressure gradient, and gravitational forces. No-slip boundary conditions are applied to arterial walls. We considered one of the most clinically available types of yttrium-90 microsphere, SIR-Spheres® with a diameter of 32 ±10µm and density of 1600 kg/m3. The simulations are conducted in the accelerating time zone as shown in Fig 2a. COMSOL Multiphysics® version 6.1 was used to solve the governing equations.
Results: The flow distribution within the simulated hepatic arterial system was influenced by the morphology and the applied boundary conditions. Figure 3 illustrates the velocity field along the symmetric plane of the domain at peak velocity (t/T = 0.15). The gastroduodenal artery (GDA) exhibited the highest flow rate among all the outlets in the domain (mean flow rate 3.0 cm3/s) but had a lower flow rate compared to the proper hepatic artery (PHA). Additionally, the daughter vessels (D1-D4) branching from the right hepatic artery (RHA) and the left hepatic artery (LHA), with mean flow rates around 1.0 cm3/s, displayed minimal variations (±0.2 cm3) in flow due to their similar sizes and bifurcation situations.
The simulations in this study aim to evaluate the impact of micro-catheter opening position along the common hepatic artery (CHA). The results indicate that strategically combining the particle release time zone and catheter opening position along CHA can direct all injected particles to desired exits with little dispersion. For example, positioning the catheter opening at the plane crossing (0, y, z) targets daughter vessel one (D1). Similarly, particles can be directed to GDA by positioning the catheter opening at the plane crossing (2, y, z). Figures 4a and 4b illustrate the spatial injection locations and particle trajectories in simulations targeting D1 and GDA, respectively.
Conclusions: Computational fluid dynamics is employed to model the transient fluid flow and particle hemodynamics in a representative hepatic arterial system during yttrium-90 microsphere radioembolization. The study highlights that adjusting the catheter opening position and time intervals of microsphere injection can guide the microspheres to preferred vessels, minimizing non-target particle delivery. While our study offers valuable insights, further research is needed to assess practical constraints towards seamless clinical translation.