Abstract
241704
Introduction: FDG functional PET (FDG-fPET) utilizes a constant infusion of FDG to capture dynamic changes in glucose metabolism underlying neuronal activity (Villien et al. 2014, Hahn et al, 2016). However, insufficient spatial resolution and sensitivity have limited the use of FDG-fPET. The NeuroEXPLORER (NX) was developed to be a next generation brain PET system with ultra-high resolution and >10x sensitivity compared to the High-Resolution Research Tomograph (HRRT). The ultra-high performance of the NX could permit measurement of activity in small brain regions, or small amounts of activity that may occur early in disease states. In this study, we evaluated the capability of the NX to measure dynamic changes in glucose metabolism throughout the visual pathway.
Methods: Three healthy subjects underwent a 90min FDG scan, consisting of a bolus and constant infusion, during which they experienced alternating 10min segments at rest and while being presented a 8Hz radial black-and-white checkerboard image presented on video display glasses (z920VGHD, Zetronix, Boston MA), beginning 20min post-injection. Each subject also underwent an anatomical MRI. One subject underwent the same PET imaging protocol on the HRRT. PET images were reconstructed with OSEM (7 iterations, 10 subsets, 0.5-mm voxels) with 1-min frames using the United Imaging Healthcare reconstruction platform and motion corrected using frame-based image registration. No spatial or temporal smoothing was applied post-reconstruction. FreeSurfer was used to define time activity curves (TACs) for each region of interest (ROI): occipital lobe, pericalcarine, lateral geniculate nucleus (LGN), frontal lobe, temporal lobe, parietal lobe, and cerebellum (Fischl 2012). PET data were analyzed by fitting TACs to a general linear model (GLM) with regressors for the baseline uptake, modeled as a quadratic fit of the total gray matter TAC, and stimulation. The %increase in uptake was calculated by calculating a ratio between the stimulation and baseline regressor estimates. Further, a combined GLM analysis was conducted by normalizing and concatenating LGN TACs for all subjects.
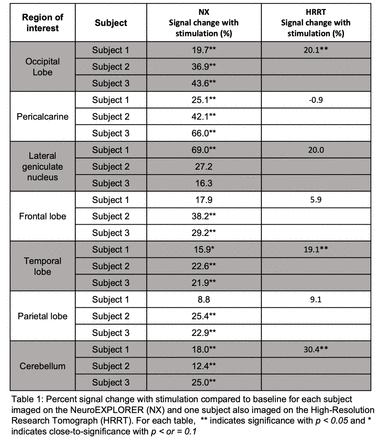
Results: Fig. 1A shows a representative NX FDG image demonstrating excellent resolution. Differences in FDG uptake corresponding to neuronal activation are measured as changes in the TAC slope. Visually, Fig. 1B/C depict slope increases during stimulation segments, relative to the baseline, in the occipital lobe and pericalcarine regions, with the overlayed respective GLM fits. Table 1 shows %signal changes, demonstrating significantly increased FDG uptake in several ROIs for all subjects; interestingly, significance in the parietal and temporal lobes was exhibited by two subjects. Significant increases in FDG uptake with stimulation in the LGN was only exhibited by one subject. Repeating the GLM analysis with normalized and concatenated LGN TACs yielded significantly increased uptake (34±3% signal change, p<0.05). Finally, Table 1B demonstrates the improved capability of the NX over the HRRT within small ROIs: pericalcarine and LGN.
Conclusions: The ultra-high resolution and sensitivity of NX FDG-fPET can potentially permit the measurement of stimulation induced changes in FDG uptake in ROIs associated with visual processing. Future directions include using ultra-high performance FDG-fPET in conjunction with unconventional stimulation paradigms to evaluate changes in glucose metabolism that occur in health and disease.