Abstract
241701
Introduction: Radio-guided surgery can reduce surgical invasiveness, improve accuracy, and minimize complications. Representative applications include breast and malignant sentinel lymph node biopsy detection, radio-guided occult lesion localization, image-guided percutaneous biopsy, and determining tumor resection boundaries. Gamma cameras visualize radioactive tracer distribution, enhancing the efficiency and precision in localizing gamma hotspots, thus making them extremely suitable for monitoring radio-guided surgeries. However, existing gamma cameras suffer from low sensitivity due to the mechanical collimator, which poses challenges for real-time surgery monitoring. To overcome this limitation, we aim to remove the collimators and develop a high-sensitivity gamma camera based on the self-collimation concept.
Methods: The key idea of self-collimation involves constructing a sparsely distributed scintillator array, where each scintillator element detects the photon itself and collimates for other elements. This makes the accumulated photon counts distribution sensitive to tracer distribution, enabling high-sensitivity gamma imaging without mechanical collimation.
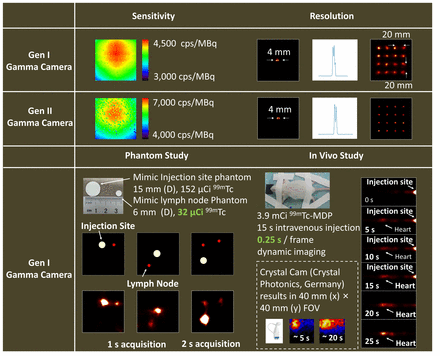
We have built two generations (Gen I and II) of self-collimation gamma cameras. Both scintillator arrays consist of cubic GAGG (Ce) scintillators and K9 glass elements. Each element measures 2.0 mm (x) × 2.0 mm (y) × 2.0 mm (z). On the x-y plane, the scintillator arrays both have external dimensions of 67.2 mm (x) × 67.2 mm (y). In the z direction, the Gen I and II arrays measure 20.0 mm and 32.0 mm, respectively. We utilize a dual-end-readout technique to measure the 3D photon interaction positions with SiPMs coupled at both ends. We integrate the scintillator array, readout electronics, power supply module, and laser positioning system into the gamma camera. Its total weight of 800 g makes it suitable for handheld applications in surgery.
We used a 99mTc point source with a diameter of 0.7 mm to measure detection sensitivity and imaging resolution. Circle paper phantoms with diameters of 6 mm and 15 mm (typical dimensions of lymph nodes and injection sites) were used to demonstrate the feasibility of gamma hotspot localization in surgery. We also monitored the activity transportation process into mice hearts following intravenous injection to validate the fast dynamic imaging capability. All data were measured within a 100 mm (x) × 100 mm (y) field of view (FOV) at a 50 mm camera-FOV distance, using a 112 – 168 keV energy window. The ML-EM algorithm was used for all image reconstructions.
Results: The average detection sensitivity of the Gen I and II gamma cameras across the whole FOV is 4092 and 5916 cps/MBq, respectively. Both cameras can separate two point sources 4 mm apart in the FOV center and resolve a 4 × 4 point source array covering the entire 100 mm (x) × 100 mm (y) FOV. Phantom studies demonstrate that we can detect a low-dose lymph node phantom with a 1-second measurement and two lymph node phantoms with a 2-second measurement. The in vivo study proves that we can monitor the activity transportation process into mice hearts in real time following intravenous injection.
Conclusions: Our study demonstrates that the proposed high-sensitivity medical gamma camera design has the potential to achieve fast and accurate low-dose gamma imaging. Further investigations are ongoing to implement more validations using the Gen II gamma camera including its performance in actual radio-guided surgeries.