Abstract
241656
Introduction: Drug resistance leads to poor stem cell-based targeted therapy in ovarian cancer (OC) patients, andthe lack of targeted markers has hindered research progress as well. Previous studies have suggestedC-X-C chemokine receptor type 4 (CXCR4) can serve as a promising marker of CSCs. We aim tovalidate the association between CXCR4 and resistant OC and to identify OC populations with highCXCR4 expression for tumor therapy under the guidance of [68Ga]Ga-Pentixafor.
Methods: Xenograft mice carrying SK-NS (control group) and SK-3rd (drug-resistant group) cells wereconstructed and confirmed by HE staining, then imaged by PET/CT with [68Ga]Ga-FAPI-04 and[68Ga]Ga-Pentixafor, respectively. CXCR4 inhibitor AMD3100 significantly impaired theself-renewal ability of CSC-like OC cells and enhanced their sensitivity to cisplatin. We thenconstructed patient-derived xenograft (PDX) models and guided their treatment grouping andefficacy monitoring by PET/CT imaging with [68Ga]Ga-Pentixafor.
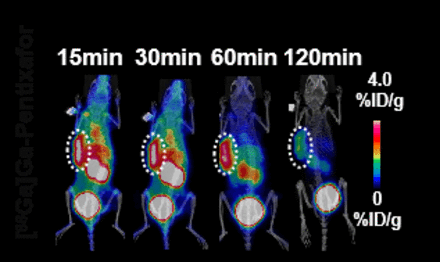
Results: IHC staining confirmed high CXCR4 expression in SK-3rd xenograft tumors, significantly higher than that of SK-NS tumors. [68Ga]Ga-FAPI-04 and [68Ga]Ga-Pentixafor PET/CT images of SK-NS and SK-3rd tumors showed SK-3rd tumors exhibited much higher and persistent radiotracer uptake of [68Ga]Ga-Pentixafor with 1.53 ± 0.15 %ID g-1 at 60min compared with these in SK-NS tumors with 0.67 ± 0.17 %ID g-1 at 60 min (P < 0.05), and there were no significant differences in tumor uptake of [68Ga]Ga-FAPI-04 between SK-NS and SK-3rd tumor models at all time points. CXCR4-targeted [68Ga]Ga-Pentixafor was highly specific in delineating CXCR4+ cell line-derived xenografts (CDXs) and patient-derived xenografts (PDXs) via PET/CT imaging, with precise tumor-targeting and persistent retention. Combination of AMD3100 and cisplatin exerted a strong, synergistic antitumor effect in CXCR4-high PDXs, but not in CXCR4-low PDXs. These results strongly suggest that CXCR4 can serve as a OC-CSC marker associated with chemoresistance and it is feasible to use[68Ga]Ga-Pentixafor PET imaging to guide decision-making for AMD3100 and cisplatin combinational therapy, paving the way for future clinical translation.
Conclusions: CXCR4 was identified as a functional CSC marker associated with chemoresistance of OC.[68Ga]Ga-Pentixafor is highly specific in delineating and visualizing CXCR4 overexpressing OCand can be used to guide OC therapy. AMD3100 in combination with cisplatin inhibits the growth ofCXCR4+ tumors. These results shed light on future OC therapy and PET imaging-guided treatmentmonitoring.