Abstract
241567
Introduction: Peptide Receptor Radionuclide Therapy (PRRT) is used to treat metastatic neuroendocrine tumors (NETs) worldwide. Multiple studies analyzing PRRT treatment response in Australian and European populations have been published. However, data from US populations is scant. This study, performed in a tertiary referral center in the Midwestern US, aims to assess the clinical, imaging, toxicity, and survival outcomes since the inception of this institution’s PRRT program.
Methods: This retrospective study included all consecutive metastatic NET patients treated with one or more doses of 177Lu-Dotatate (Lutathera®) between March 2019 and February 2023, with a minimum follow-up period of 10 months. Primary endpoints included tumor response assessment using metabolic imaging tumor volume and response evaluation criteria in solid tumors version 1.1 (RECIST v1.1), progression free survival (PFS), overall survival (OS), and safety profile. Secondary endpoints included analyzing trends of primary tumor location, metastatic sites, and tumor markers favoring disease progression.
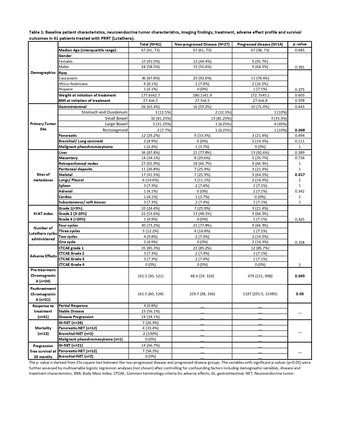
Results: A total of 41 patients (median age (interquartile range, IQR): 67 years (61,73); 58.5% male; 87.8% Caucasians) were studied: 26 (63.4%) gastrointestinal-NET, 12 (29.2%) pancreatic-NET, 2 bronchial-NET, and one malignant pheochromocytoma. Most (32 out of 33, 97%) of the patients with known Ki-67 index belonged to WHO grade 1 or 2 NET, with Ki-67 less than 20%. 30 (73.2%) patients received all four cycles, five received three, four received two, and two patients received one cycle. The median follow-up period (IQR) was 40 (27.5, 48) months. 23 (56.1%) patients demonstrated stable disease, 4 (9.8%) showed partial response, and 14 (34.1%) patients had disease progression. 13 (31.7%) patients died during the follow-up period. As over half of the patients were alive and with no disease progression, median progression free survival and median overall survival were not reached at the end of follow-up period. 38 (92.6%) patients had grade 1 or 2 adverse effects per the common terminology criteria for adverse effects, and 3 patients had grade 3 adverse effects requiring hospital admission and/or blood product transfusion. No treatment related renal or liver dysfunction, myelodysplastic syndrome, or leukemia was reported. Transient lymphopenia and hyperglycemia were noted in 33 (80.5%) and 24 (58.5%) patients, respectively. Multivariable logistic regression analysis demonstrated significantly higher disease progression in patients with skeletal metastases (p=0.038, Odds Ratio (95% confidence interval): 4.28 (1.09,16.8)). Higher pre-treatment (median: 479 versus 48.9, p=0.045) and post-treatment (median: 1187 versus 103.7, p=0.03) chromogranin A (CgA) levels were noted in the progressed compared to non-progressed disease group. Presence of a primary colonic NET favored disease progression (p=0.049) compared to primary small bowel NET.
Conclusions: These results support the role of PRRT for favorable survival outcomes and toxicity profile in patients with metastatic NETs in a US-based population. All treatment related adverse effects were transient and did not lead to treatment discontinuation. Presence of skeletal metastases was associated with disease progression, and presence of colonic primary NET favored disease progression compared to small bowel NET. Presence of higher pretreatment CgA levels trended towards disease progression. These findings need to be further investigated in larger studies.