Abstract
241563
Introduction: Overexpressing in many solid malignancies, gastrin-releasing peptide receptor (GRPR) is a promising cancer imaging marker and therapeutic target. The development of GRPR-targeted ligands is mainly based on the sequence of bombesin, an exogenous GRPR ligand. Some GRPR-targeted tracers have been translated into the clinic, but there are still some limitations, such as high pancreas uptake and low in vivo stability. Our group previously reported a 68Ga-labeled bombesin analog, [68Ga]Ga-TacsBOMB2 (68Ga-DOTA-4-amino-1-carboxymethylpiperidine(Pip)-[D-Phe6,Leu13ψThz14]Bombesin(6-14)), which showed low pancreas uptake (Wang L, et al. Molecules. 2022; 27: 3777). In this study, we developed four TacsBOMB2 derivatives with unnatural amino acid substitutions, and evaluated their potential for detecting GRPR-expressing tumors with positron emission tomography (PET).
Methods: LW01158LW01160 (DOTA-Pip-[D-Phe6,NMe-His12,Leu13ψThz14]Bombesin(6-14)), LW01186 (DOTA-Pip-[D-Phe6,a-Me-Trp8,Tle10, Leu13ψThz14]Bombesin(6-14)), and LW02002 (DOTA-Pip-[D-Phe6,Tle10, N-Me-Gly11, Leu13ψThz14]Bombesin(6-14)) were synthesized using Fmoc peptide chemistry on solid phase. Gallium (natGa and 68Ga) complexation was conducted in acetate (0.1 M, pH 4.5) and HEPES (2 M, pH 5.0) buffers, respectively, and purified via HPLC. GRPR binding affinities and agonist/antagonist characterizations were determined by in vitro competition binding and calcium release assays, respectively. Imaging and biodistribution studies were conducted in PC-3 tumor-bearing NRG mice at 1 h post-injection (pi). In vivo stability was conducted in NRG mice at 15 min pi.
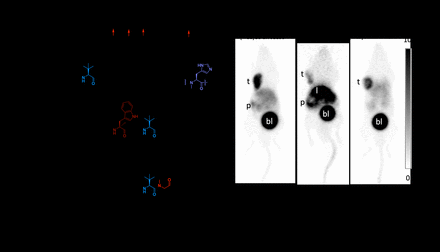
Results: LW01158, LW01160, LW01186, and LW02002 were synthesized in 7.7-32 % yields, and their non-radioactive Ga-complexed standards were synthesized in 76-81% yields. Binding affinities (Ki) of Ga-LW01158, Ga-LW01160, Ga-LW01186, and Ga-LW02002 were 5.11±0.47, 187±17.8, 6.94±0.95, and 11.0±0.39 nM, respectively. All Ga-LW01158, Ga-LW01186, and Ga-LW02002 were confirmed to be GRPR antagonists via calcium release assay. [68Ga]Ga-LW01158, [68Ga]Ga-LW01186, and [68Ga]Ga-LW02002 were obtained in 16-61 % decay-corrected radiochemical yields with >92% radiochemical purity. Both [68Ga]Ga-LW01158 and [68Ga]Ga-LW02002 clearly visualized PC-3 tumor xenografts in PET images at 1 h post-injection, and were excreted mainly via the renal pathway. [68Ga]Ga-LW01186 showed very high uptake in liver, and muck lower uptake in PC-3 tumor xenografts. [68Ga]Ga-LW01158 had the highest tumor uptake (11.2±0.65%ID/g), followed by [68Ga]Ga-LW02002 (8.32±1.20 %ID/g) and [68Ga]Ga-LW01186 (5.76±0.71 %ID/g). [68Ga]Ga-LW01186 showed the highest liver uptake (23.5±1.81 %ID/g), while the values for both [68Ga]Ga-LW01158 and [68Ga]Ga-LW02002 were only 4.33±0.22 and 1.06±0.24 %ID/g, respectively. [68Ga]Ga-LW01186 also showed the highest pancreas uptake at 1 h post-injection (14.2±2.29 %ID/g), followed by [68Ga]Ga-LW01158 (12.0±1.41 %ID/g) and [68Ga]Ga-LW02002(2.36±0.36 %ID/g). There were 80.7±1.57% of [68Ga]Ga-LW01158, 76.5±2.91% of [68Ga]Ga-LW01186, and 76.6±7.00% of [68Ga]Ga-LW02002 remaining intact in plasma at 15 min pi, which is comparable with that of [68Ga]Ga-TacsBOMB2 (83.3±1.45%). No intact tracer was detected in urine samples for either [68Ga]Ga-LW01158 or [68Ga]Ga-LW02002, while 43.6±3.46% of intact [68Ga]Ga-LW01186 was detected in urine samples at 15 min pi.
Conclusions: Replacing Val10, Gly11, and Trp8 in [D-Phe6,Leu13ψThz14]Bombesin(6-14) sequence with Tle10, NMe-Gly11and ,a-Me-Trp8, respectively, retains the good binding affinity toward GRPR, antagonist characteristic, and good in vivo stability. However, the substitution of His12 by N-Me-His12 results in a significantly inferior binding affinity to GRPR. With good tumor uptake and tumor-to-background contrast, [68Ga]Ga-LW01158 is promising for detecting GRPR-expressing tumors with PET.