Abstract
241524
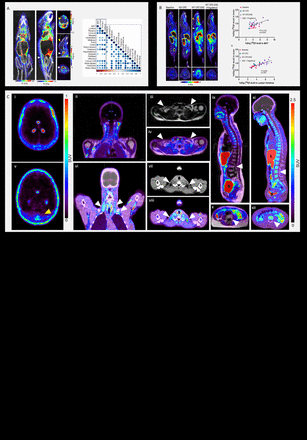
Introduction: Adipose tissue (AT) is a heterogenous organ with a complex function crucial for diverse processes including energy storage, endocrine signaling and immunomodulation. Mitochondria-rich brown and brown-like adipose tissues have garnered considerable interest due to their role in metabolism and potential therapeutic applications in diabetes and obesity. The connection between brown fat and immunity has been scarcely studied, but new studies provide evidence that brown adipose tissue (BAT) affects not only metabolism but also systemic immune responses. In this study, we use [18F]F-AraG, a mitochondrial metabolic tracer capable of tracking activated lymphocytes and adipocytes simultaneously, to demonstrate a link between activated adipose tissue and neuroinflammation.
Methods: We investigated two preclinical models that are associated with neuroinflammation, GL261 glioblastoma (GBM) model, and Cuprizone (CPZ) and experimental autoimmune encephalomyelitis (EAE) model for multiple sclerosis (MS). Two groups of mice with intracranial GBM tumors (n=10 total), one treatment-naïve and the other treated with anti-PD-1/CTLA-4 therapy, were longitudinally imaged using [18F]F-AraG. The mice with MS were imaged before the disease induction (n=10), after CPZ treatment at week 3 (n=5) and post immunization at week 7 (n=10). Mice (n=10) treated with immunosuppressant fingolimod were imaged with [18F]F-AraG to evaluate the effect of reduced intracerebral inflammation on activation of adipose tissue. The link between neuroinflammation and adipose tissue was also investigated in a cohort of nineteen post-acute COVID-19 human subjects.
Results: In both untreated and immunotherapy treated GBM-affected mice, activation of brown fat as well as bone marrow adipose tissue coincided with immune activation and neuroinflammation. Correlations found between signal in the AT and various areas in the brain in treatment-naïve mice were absent in mice treated with immunotherapy, indicating the effect of therapy on the AT-neuroinflammation relationship. Imaging of the CPZ-EAE model demonstrated that AT activation coincides with intracerebral T cell infiltration. Treatment with immunosuppressant fingolimod led to a significant decrease in [18F]F-AraG accumulation in BAT. Interestingly, in four post-acute COVID-19 subjects we observed co-occurrence of neuroinflammation and activated BAT, a phenomenon absent in pre-pandemic controls.
Conclusions: Leveraging [18F]F-AraG’s capacity to simultaneously track activated lymphocytes and adipocytes, we demonstrate in GBM and MS models the correlation between intracerebral immune infiltration and changes in brown- and bone marrow adipose tissue, and show initial evidence that a neuroinflammation-adipose tissue link may also exist in humans. This study proposes the concept of an intricate immuno-neuro-adipose circuit, and highlights brown- and bone marrow adipose tissue as an intermediary in the communication between the immune and nervous systems.