Abstract
241388
Introduction: The fields of radio-guided surgery and interventional nuclear medicine are advancing with a range of innovative technologies. Crucial among them are surgical probes capable of efficiently detecting β+ emitters to localize tumors identified in PET images. Various methods have been proposed for F-18 radio-guidance within the body, including the detection of 511 keV annihilation photons using electronically or mechanically collimated probes. However, the widespread adoption of these techniques faces challenges such as the lack of precise directional capabilities and inadequate sensitivity, coupled with the bulkiness and weight of the probes.
An unresolved inquiry centers on establishing the optimal signal-to-background ratio attainable with an idealized probe. Additionally, the potential advantages that radio-guided surgery might derive from detectors based on Compton scattering are still uncertain. Initially, we employed the Monte Carlo simulation platform GATE to address the first question. Subsequently, our attention turned to the examination of a small Compton-angles collimation prototype probe, resulting in the development of an initial proof-of-concept demonstrator. The performance of the small Compton-angle collimation probe was then evaluated against a commercial probe employing mechanical collimation, with a focus on sensitivity and directionality.
Methods: A phantom mimicking a PET/CT Well Counter Correction (WCC) phantom, resembling a Plexiglas cylinder was simulated. The volume's activity concentration was set to 3.55 MBq/L, representing the injection of 20 MBq of a positron-emitting radioisotope (Fluorodeoxyglucose) into the WCC phantom, simulating healthy tissue one hour after injection. To represent a lymph node or a small tumor with high uptake, a spherical volume of 1 cm diameter was placed in the phantom 5 mm from the surface. The phantom activity concentration was varied from 1 to 12 Standard Uptake Value (SUV). A perfectly collimated probe was simulated as a 1 cm diameter cylinder, with a sensitivity to any impinging gamma ray from its direct front.
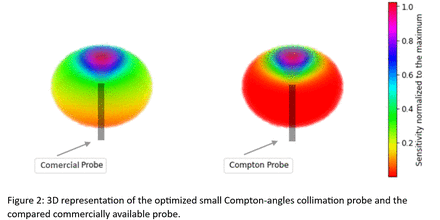
A Compton-angle collimation prototype was built with two Teflon-wrapped crystals (1 cm diameter, 1.5 cm and 3 cm length), coupled to SiPMs, and protected by a 3.5 mm tungsten layer. The distance between detectors allows detecting gamma rays at angles equivalent to a commercialized high-energy probe (monolithic crystal collimated with thick tungsten, total diameter 2.1 cm). The angular sensitivity was measured using a germanium point source. Experimental replication with the simulated WCC phantom allowed to assess the small angles-Compton probe's ability to measure regions with abnormally high SUV.
Results: Monte Carlo simulations indicated that an idealistic probe would yield a signal to background ratio of approximately 25% for 1 cm diameter tumor positioned 5 mm under the skin and a SUV of 2. Unlike Tc-99m, which is injected under the skin for sentinel nodes detection, FDG injection through the venal system generates a strong radioactive background due to its fixation by cells consuming glucose.
The small Compton-angles collimated probe prototype demonstrated improved directionality compared to the mechanically collimated commercial probe (Figure 2). Realistic measurements in the WCC phantom showed the feasibility of distinguishing a SUV of 4 from the background of a concentration of 3.55 MBq/L.
Conclusions: The implementation of small Compton-angles collimation shows promising results, especially in enhancing directionality for improved tumor detection while minimizing probe size and weight. Monte Carlo simulations showed a significant effect of background activity on the measured signal, which could be addressed by enhancing the probe sensitivity by upgrading the detector crystal material and geometry.