Abstract
241311
Introduction: To evaluate the clinical impact of 16α-18F-fluoro-17β-estradiol (18F-FES) PET/CT on the management of breast cancer patients.
Methods: Study subjects were identified from the database of a prospective trial for post-marketing surveillance of 18F-FES between 2021 and 2023, who were suspected or known to have recurrent or metastatic estrogen receptor-positive breast cancer based on routine standard workup. The primary outcome was to assess the impact of 18F-FES PET/CT on management in terms of the proportion of cases in which the clinician’s management plan changed based on 18F-FES PET/CT compared to that based on standard workup. Planned management before 18F-FES PET/CT, and actual management after 18F-FES PET/CT were assessed by two experienced medical oncologists via medical chart review. A five-point questionnaire were provided to evaluate the value of 18F-FES PET/CT to management planning.
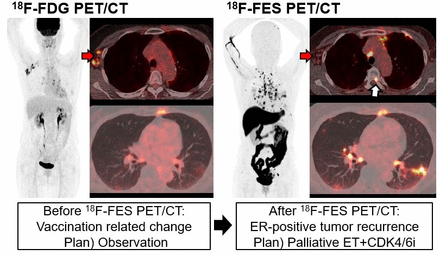
Results: Of the total 348 patients included, 121 (35%) experienced a change in management after 18F-FES PET/CT. In 138 (40%) patients, 18F-FES PET/CT supported the existing management decision while did not change management. The intention-to-treat and inter-modality changes were observed in 81 (23%) and 83 patients (24%), respectively. Higher rate of change was observed when lesions were FES-negative (45% [38/85]), compared to FES-positive (26% [44/172]) or mixed FES-positive/negative (34% [31/91]). Regarding clinical indications, the highest rate of change was shown when evaluating origins of metastasis in case of double primary cancers (50%).
Conclusions: 18F-FES PET/CT significantly modified management in recurrent or metastatic breast cancer, serving as a crucial imaging modality in clinical practice.