Abstract
241276
Introduction: PURPOSE: 177Lu-PSMA-617 radioligand therapy (RLT) is approved for patients with metastatic castration-resistant prostate cancer (mCRPC) who progress on at least one taxane and one androgen receptor pathway inhibitor. Elevated PSMA levels are vital for effective RLT doses and positive tumor responses. Multiple trials are ongoing to study PSMA RLT in the prechemotherapy setting (PSMAfore, SPLASH and ECLIPSE). We aimed to measure the impact of taxane chemotherapy on PSMA expression as visualized on PSMA PET uptake.
Methods: METHODS: We retrospectively identified mCRPC patients treated with at least two cycles of taxane chemotherapy, who had PSMA PET performed prior to and after completion of chemotherapy. Response to chemotherapy was defined as a PSA decline of 50% from baseline; responders whose PSA was still within 25% of nadir at the time of their post-treatment PSMA PET were classified as ongoing responders. Non-responders were defined as those patients whose PSA increased by more than 25% of baseline while on chemotherapy, and the remainders of patients were classified as having stable disease. PSMA-avid lesions were segmented using SUV of 3 (or 1.5 times the liver for liver lesions) to measure SUVmax, SUVmean, PSMA avid tumor volume and total lesion PSMA-expression. PET parameters were compared between pre- and post-chemotherapy PET based on PSA response.
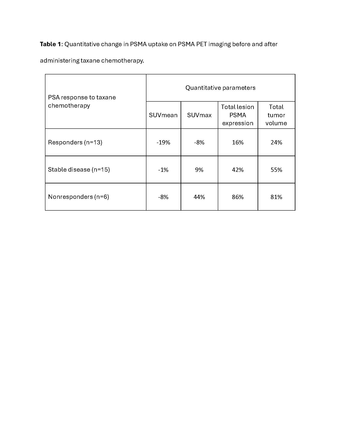
Results: RESULTS: 34 mCRPC patients were identified including 94% with osseous, 71% with lymph nodes and 56% with visceral metastatic lesions. Thirteen (38%) patients achieved a PSA response to chemotherapy, including 2 with ongoing PSA response at the time of their post-treatment PET; 15 (44%) patients achieved stable disease, and 6 (18%) were non-responders to chemotherapy. When compared to baseline PET, 64% patients showed decrease in SUVmean, out of which 5 patients had their SUVmean drop below 10 after chemotherapy. Among responders, SUVmean decreased by 19%, while those with an ongoing PSA response had a decrease of 21%. By comparison, SUVmean decreased by 8% in non-responders, and 1% in patients with stable disease. In the responders, SUVmax decreased by an average of 8% while the SUVmax increased by an average of 44% in non-responders, and 9% in patients with stable disease.
Conclusions: CONCLUSION: Response to chemotherapy resulted in lower SUVmean and SUVmax after taxane chemotherapy when compared to pre chemotherapy values. The subgroup of patients with an ongoing PSA response at the time of their post-chemotherapy PET had the biggest fall in SUVmean. Our results suggest that taxane chemotherapy is associated with decreased PSMA expression on PET imaging.