Abstract
241255
Introduction: Recent advances in immunotherapy with immune checkpoint inhibitors (ICI) have changed the prognosis of patients with advanced and/or metastatic melanoma. However, a significant number of patients will not benefit from immunotherapy due to therapy resistance mediated by the the tumor microenvironment (TME). Tumor associated macrophages (TAMs) are of particular importance in this setting. Alternatively activated M2-polarized CD206-positive TAMs are strongly immunosuppressive, and their presence in the TME was reported to be associated with poor patients’ outcomes. We describe here a first human SPECT/CT study to visualize CD206-positive M2-like TAMs in patients undergoing immunotherapy with ICI (NCT04663126).
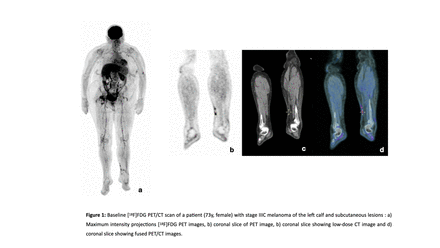
Methods: In this prospective single-institution, open label, non-randomized, preliminary study we aimed to assess the local presence of CD206-positive, M2-like TAMs via SPECT/CT imaging using the CD206-targeted tracer [99mTc]Tc-Tilmanocept (Lymphoseek™, NAVIDEA Biopharmaceuticals, OH, USA) in advanced melanoma patients at baseline or presenting with progressive disease under ICI treatment. Eligible patients had previously undergone [18F]FDG PET/CT within 28 days prior inclusion with measurable uptake in at least 3 suspicious lesions. They all received an intravenous injection of 350 MBq (+/- 15%) [99mTc]Tc-Tilmanocept and underwent a dynamic scintigraphic acquisition followed by a whole-body planar acquisition and a quantitative SPECT/CT at 1 and 3 hours after injection. The following quantitative parameters on SPECT/CT were measured: SUVmax, SUVpeak, SUVmean, Metabolic tumour volume (MTV) and Tumor Lesion Activity (TLA) on suspicious target lesions visible on [18F]FDG PET/CT. A spherical volume of interest was used for healthy tissues to define ratios between lesion SUVmax and healthy tissues SUVmean. Those measures were compared to SUVmax, SUVpeak, SUVmean, MTV and Tumor Lesion Glycosis (TLG) calculated for baseline [18F]FDG PET/CT imaging and correlated with CD206 and CD8 cells densities on clinically indicated biopsy results as well as tumor response on standard of care imaging at 3 months under ICI treatment according to EORCT criteria and follow-up.
Results: In total, 5 patients were included in this study. Dynamic acquisition did not show any increase during the initial lesion perfusion. Lesion signal on SPECT/CT imaging was stable at 1- and 3-hour post-injection without significant differences for SUVmax/peak/mean, MTV and TLA measured on [99mTc]Tc-Tilmanocept SPECT at 1- and 3-hour (all p>0.15). Significant and strong correlations were seen between quantitative parameters measured on SPECT/CT at 1- and 3-hour respectively and baseline [18F]FDG PET/CT. There was a borderline significant, but strong correlation between CD206 cell densities using linear regression on patients’ biopsies and MTV measured [99mTc]Tc-Tilmanocept SPECT/CT at 1-hour [R2 = 0.626, p = 0.061]. Additionally, we found that the ratio between SUVmax target lesion/ SUVmean fat-tissue calculated on [99mTc]Tc-Tilmanocept SPECT/CT at 1-hour was significantly associated with tumor response at 3 months with an average value of 11.2±1.3 non-responder patients versus 5.3±2.8 otherwise (p=0.005) and with poorer outcomes during the follow-up (p=0.026).
Conclusions: This pilot trial using [99mTc]Tc-Tilmanocept SPECT/CT imaging for the detection of M2-like TAMs in the TME has yielded encouraging preliminary results for potential clinical routine application. We report significant correlations between lesion signal on [99mTc]Tc-Tilmanocept SPECT/CT imaging, CD206+ TAMdensities in biopsies as well as local tumor response and outcome. These preliminary pilot data provide the first-in-human evidence that CD206 functional imaging holds promise in predicting tumor responses to immunotherapy, potentially paving the way for a more tailored approach in the future use of immunotherapy.