Abstract
241192
Introduction: A number of pathological conditions are associated with the aggregation of proteins, which form insoluble amyloid deposits in body tissues. Such deposits can be local, where aggregates remain in the organ of origin, or systemic, where fibrillar aggregates reach the bloodstream and deposit in other tissues of the body. In patients with T2DM, amyloid deposits can often be found in the pancreas, as amylin has a highly amyloidogenic characteristic, and in non-physiological conditions, increased production of this hormone promotes its incorrect folding, resulting in the formation of amyloid deposits in the form of toxic oligomers and/or insoluble fibers in the pancreas, which can be deposited in other body tissues. However, one of the main challenges is to diagnose amyloidosis, which is often diagnosed late. Nuclear medicine, through the use of radiopharmaceuticals with high specificity, has been widely used in the diagnosis of numerous diseases, including the diagnosis of amyloidosis.
Methods: After human amylin (hA) aggregation standardization tests; Dot blot, Transmission Electron Microscopy analysis, and induction of animals with aggregated hA via intravenous (IV) and intraperitoneal (IP) routes, we performed the labeling of an anti-oligomer antibody and aggregated and soluble hA with Technetium-99m to evaluate biodistribution via IV and IP by imaging, organ counting and histopathological analysis in male Wistar rats (70 - 90 days). ANOVA analysis of variance followed by multiple comparisons test with p<0.05.
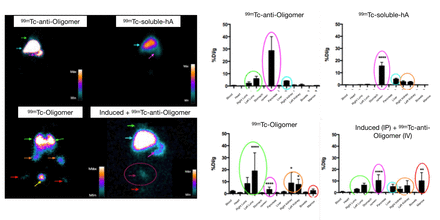
Results: Our results showed a labeling efficiency and stability above 90%. Furthermore, the scintigraphic images of the groups that received aggregated amylin showed a higher uptake at the injection site of hA aggregates, lung topography (18.9 ± 15.18 %DI/g), bone marrow (10.05 ± 7.17 % DI/g) and kidneys (8.8 ± 8.90 %DI/g), corroborating the histopathological findings, with inflammatory infiltrate and thickened septa in the lungs, calcified structures and desquamation inside the renal tubules. Figure 1 shows the scintigraphic images of the groups that received aggregated amylin, unlike the group that received only the labeled antibody, which showed higher uptake at the injection site of hA aggregates, lung topography (18.9 ± 15.18 %DI/g), bone marrow ( 10.05 ± 7.17% DI/g) and kidneys (8.8 ± 8.90% DI/g), being higher in the group that received oligomers labeled with 99mTc via IV, proving that hA is partly excreted by the kidneys, as well as its aggregated form, corroborating our histopathological findings. Figure 2 shows representative images of lung histology from normal animals (CTRL), hA animals injected with soluble human amylin and induced, injected with aggregated human amylin via IV and IP. These are interesting results since there was uptake in organs that, through histological analysis, can be correlated with the activity of the immune system in the tissue in an attempt to eliminate or neutralize soluble or aggregated amylin, causing tissue inflammation.
Conclusions: The labeled antibody was able to identify hA deposits in the animal's body tissues through scintigraphy, being a promising radiopharmaceutical for diagnosing amyloidosis triggered by hA. However, more studies need to be performed.