Abstract
241098
Introduction: The increase in γ-secretase activity in the brain of Alzheimer disease (AD) patients and animal models of AD position this enzyme as a potential biomarker of the disease. However, measuring γ-secretase activity both in vitro and in vivo presents challenges due to the limited fraction of catalytically active γ-secretase complexes and the lack of correlation between enzymatic activity and the quantity of the catalytic subunit, presenilin. In this study, we radiolabeled an analog of SGSM-15606, referred to as [11C]1 (IC50 of Aβ42 = 6.5 nM), with carbon-11 and evaluated its potential as a γ-secretase PET probe in a three-stage validation process. Initially, we assessed the biodistribution, metabolism, and blood brain barrier (BBB) crossing capacity of [11C]1 in wild type (WT) mice and simultaneously gauged its potential to detect γ-secretase levels in AD mouse models through PET-CT imaging studies. Secondly, we undertook preliminary PET-MR imaging studies with [11C]1 in nonhuman primates (NHPs) to evaluate its brain permeability, regional distribution, and specific washout kinetics. Thirdly, we evaluated the [11C]1's capability for longitudinal in vivo assessment of γ-secretase expression in AD mouse models. Our findings indicate that using [11C]1 for PET neuroimaging effectively visualizes γ-secretase in brain regions of preclinical animals, underscoring its significant potential for clinical studies in AD patients.
Methods: [11C]1 was radiolabeled through 11C-methylation. Metabolism studies were performed on blood and brain samples of wild type (WT) mice. Biodistribution studies were performed in WT mice using dynamic PET-CT imaging. Specific binding was demonstrated by in vivo PET imaging blocking studies in WT mice. To evaluate the translational potential of [11C]1 as a possible step forward toward human studies, we carried out preliminary imaging studies in NHPs. The brain permeability, regional distribution, and specific washout kinetics were evaluated by dynamic PET-MR imaging. Longitudinal PET imaging of γ-secretase was conducted in 5xFAD mice at 3, 6, and 12 months of age and compared to age-matched control animals.
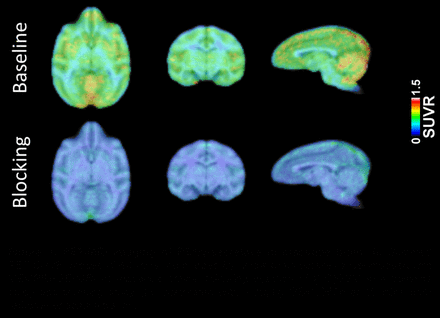
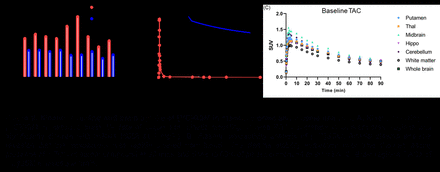
Results: [11C]1 was produced in sufficient radiochemical yield and molar activity for the use in PET imaging. Metabolism and biodistribution studies confirmed sufficient stability in vivo, the ability of [11C]1 to cross the blood brain barrier (BBB) and slow washout from the brain. Blocking studies confirmed specificity of the binding. The PET-MR imaging in NHPs demonstrated high brain uptake (0.5 - 1.5 in SUV) (Figure 1). Notably, relatively higher uptake was observed in regions such as hippocampus and cortex. Blocking study in NHPs showed decreased SUVR (normalized by whole brain uptake) in brain regions. The kinetic modeling results showed [11C]1 was expressed across different brain regions, and the levels in hippocampus and cortex displayed high levels and also with reduced VT in blocking study in brain regions, indicating the specific binding of [11C]1 (Figure 2). Longitudinal PET studies showed increased levels of γ-secretase in the cerebral cortex, hippocampus, striatum, thalamus, cerebellum and brain stem in aged AD mice compared to WT littermates.
Conclusions: The γ-secretase inhibitor [11C]1 crosses the BBB and is slowly washed out of the brain of WT mice. Comparison between AD and WT mice shows accumulation of the radiotracer in the AD-affected areas of the brain over time during the early disease progression. The imaging data in NHPs demonstrated that [11C]1 has a great potential for future study in AD patients. These results suggested that PET neuroimaging using [11C]1 allowed us to visualize γ-secretase in brain.