Visual Abstract
Abstract
This study aimed to compare the efficacy of [18F]F-choline PET/CT with conventional imaging for staging and managing intermediate- to high-risk prostate cancer (PCa). The primary objective was to assess the ability of PET/CT with [18F]F-choline to identify lymph node and systemic involvement during initial staging. Secondary objectives included evaluating the impact of [18F]F-choline PET/CT on unnecessary local treatments and assessing the safety of [18F]F-choline agents. Additionally, the study aimed to analyze recurrence-free survival and overall survival 5 y after randomization. Methods: A prospective controlled, open, randomized multicenter phase III trial involving 7 Italian centers was conducted. Eligible patients with intermediate- to high-risk PCa were randomized in a 1:1 ratio. Two groups were formed: one undergoing conventional imaging (abdominopelvic contrast-enhanced CT and bone scanning) and the other receiving conventional imaging plus [18F]F-choline PET/CT. The study was terminated prematurely; however, all the endpoints were thoroughly analyzed and enriched. Results: Between February 2016 and December 2020, 256 patients were randomly assigned. In total, 236 patients (117 in the control arm and 119 in the experimental arm) were considered for the final assessment. In the experimental arm, the sensitivity for lymph node metastases, determined by final pathology and serial prostate-specific antigen evaluations, was higher than in the control arm (77.78% vs. 28.57% and 65.62% vs. 17.65%, respectively). The [18F]F-choline was tolerated well. The use of [18F]F-choline PET/CT resulted in an approximately 8% reduction in unnecessary extended lymphadenectomy compared with contrast-enhanced CT. Additionally, [18F]F-choline PET/CT had a marginal impact on 5-y overall survival, contributing to a 4% increase in survival rates. Conclusion: In the initial staging of PCa, [18F]F-choline PET/CT exhibited diagnostic performance superior to that of conventional imaging for detecting metastases. [18F]F-choline PET/CT reduced the rate of unnecessary extensive lymphadenectomy by up to 8%. These findings support the consideration of discontinuing conventional imaging for staging PCa.
Prostate cancer (PCa) remains the most prevalent malignancy among men, with metastatic progression contributing significantly to patient mortality (1). Current therapeutic decision-making regarding primary-tumor treatment depends on parameters such as prostate-specific antigen (PSA) value, clinical tumor stage, and biopsy Gleason score (2). However, the existing imaging tools and nomograms fall short in accurately staging early pelvic lymph node, bone, and distant metastases.
CT and MRI exhibit limited sensitivity and specificity in detecting small-volume metastatic disease, whereas whole-body bone scans may overlook early bone marrow metastases (3). Nonetheless, contrast-enhanced CT (ceCT) and whole-body bone scans are widely used as the standard initial diagnostic imaging modalities for staging localized PCa. Retrospective data suggest that PET imaging using [11C]choline, [18F]F-choline, or [18F]fluoride is more accurate than conventional methods in detecting lymph node and bone metastases, both for staging and for restaging purposes (4–7). However, the promising accuracy of radiolabeled choline PET/CT in identifying lymph node involvement during primary staging is tempered by several limitations. These include low sensitivity for micrometastases, inherent nonspecificity of choline uptake, retrospective study designs, limited comparative data with conventional imaging, absence of cost-effectiveness evaluations, and an uncertain impact on patient management. Consequently, current European and American guidelines do not recommend radiolabeled choline PET/CT in the initial staging of newly diagnosed PCa.
Recent advancements, notably radiolabeled prostate-specific membrane antigen (PSMA), have revolutionized PCa imaging, including the initial staging process, as evidenced by the proPSMA trial (8). Nonetheless, standardization efforts are under way to enhance the reproducibility and applicability of PSMA PET across different centers. Despite PSMA PET’s superior accuracy in detecting metastatic spread and high-risk PCa, its ability to improve clinically relevant outcomes in advanced PCa remains unproven, and it cannot yet replace extended pelvic lymph node dissection (2). Currently, only preliminary data are available, from Djaïleb et al. (9).
Radiolabeled choline PET/CT remains of interest and is still used worldwide in the diagnostic management of PCa patients. In light of this, a prospective randomized multicenter study was conceived at a time when PSMA imaging was not widespread. This study aimed to compare PET/CT using [18F]F-choline with conventional imaging for staging and managing intermediate- to high-risk PCa. The primary objective was to assess the efficacy of [18F]F-choline PET/CT in identifying lymph node and systemic involvement in these patients at initial staging. Secondary objectives included evaluating the impact of [18F]F-choline PET/CT on unnecessary local treatments and assessing the safety of [18F]F-choline agents. An additional objective was to evaluate recurrence-free survival (RFS) and overall survival (OS) after 5 y from randomization.
MATERIALS AND METHODS
Study Design and Participants
This was a prospective controlled, open, randomized phase III trial involving 7 centers in Italy (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org).
Patients were eligible if they had intermediate- or high-risk PCa according to version 2.2013 of the National Comprehensive Cancer Network–National Comprehensive Cancer Network classification (e.g., PSA ≥ 10 ng/mL and Gleason score ≥ 7, Gleason score ≥ 8 with any PSA value, cT2c-T3 with any PSA value, or PSA ≥ 20 ng/mL with any Gleason score), were older than 18 y, were candidates for radical prostatectomy and lymphadenectomy or radiotherapy, and had accessible follow-up information.
Conversely, excluded from the study were patients with a previous history of cancer, metastases to lymph nodes, or metastases to other sites already confirmed by a histopathologic examination; patients who were candidates for hormone therapy as primary treatment because of their general health condition; patients who had already been treated with hormone therapy or previous radiotherapy; and patients who had psychiatric disorders contraindicating PET/CT examination (Supplemental Table 2).
The study protocol was performed in accordance with the Declaration of Helsinki, and all participants provided written informed consent before undergoing any study procedures. The study was approved by the Ethical Committee of the Veneto Institute of Oncology in November 2013 (EudraCT 2013-002511-99; approval 2013/54).
Randomization
Eligible patients were randomized using a 1:1 ratio. The study comprised 2 groups of patients: one was studied by conventional imaging (abdominopelvic ceCT and bone scanning), whereas the other was evaluated by conventional imaging plus [18F]F-choline PET/CT (Supplemental Fig. 1). During the urologic visit, the patient’s eligibility for the study was determined by collection of demographic information, medical history, blood serum results, and histopathologic data from the PCa biopsy. A web-based database (REDCap) was used for the randomization and for collecting all data.
Study Procedures
The patients underwent the imaging procedures within 1 mo.
ceCT of Abdomen and Pelvis
ceCT imaging was performed after contrast medium injection. For the lymph node staging, the short axis was measured in all lymph nodes using a cutoff of 10 mm. For distant metastases, any abnormalities in the skeleton, liver, lung, or adrenal gland were considered. Any bone metastases were classified as osteolytic, osteosclerotic, or mixed.
Bone Scanning
Whole-body bone scan images were obtained in anterior and posterior views, 2 or 3 h after 99mTc-methyldiphosphonate injection. The results were considered positive if there were solitary or multiple asymmetric areas of uptake not considered to be due to recent trauma or an osteoarticular degenerative disease. Conversely, negative findings were defined as no abnormal uptake outside the physiologic distribution of the tracer.
PET/CT
Whole-body PET/CT was performed from the vertex to the proximal femur at 6–7 bed positions (2–3 min per position), 60 min after intravenous administration of the tracer (3 MBq/kg dose of [18F]F-choline). A low-dose whole-body CT scan (with no contrast enhancement; 140 kV, 80–120 mA) was used for attenuation correction and for anatomic localization of the sites of disease. A lymph node metastasis was defined as focal tracer uptake in the abdominopelvic lymph nodes (including nodes with a diameter < 10 mm), subsequently confirmed by equivalent CT images. Weak [18F]F-choline uptake (<liver uptake) in inguinal and mediastinal lymph nodes was considered to be reactive lymphadenitis and not pathologic (10). A distant metastasis was defined as focal tracer uptake coinciding with bone, liver, lung, or adrenal gland, whether or not it correlated with the morphologic pattern on CT (i.e., bone marrow lesions). The [18F]F-choline PET/CT images were jointly interpreted at each center involved in the trial by 2 specialists trained to perform PET/CT imaging. Intra- and interassay variations were assessed by a masked review of all [18F]F-choline images by an independent nuclear medicine specialist with specific training on [18F]F-choline PET/CT images.
Gold Standard
The standard of reference was the histopathologic data from patients who underwent radical prostatectomy and lymphadenectomy (limited, extended, or superextended). Moreover, in patients who received radiotherapy, chemotherapy, or hormonal therapy, follow-up data were considered, including clinical evaluation, serial biochemistry, and conventional or [18F]F-choline PET/CT imaging for at least 6 mo from the primary treatments. Biochemical recurrence was defined as recurrence after an increase in serum PSA level above 0.2 ng/mL or a serum PSA level above nadir plus 2.0 ng/mL after definitive radiotherapy (11).
Outcome Measures
The primary aim was to test the diagnostic performance of [18F]F-choline PET/CT on a per-patient basis by calculating the method’s sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. For lymph node metastases, the obturator, external and internal iliac, pararectal, retroperitoneal (lumbar–aortic), and deep inguinal regions were considered. For bone metastases, the following sites were considered separately: pelvis, thoracic and lumbar spinal column, ribs, femurs, humerus, skull, and so on. For distant metastases, the considered sites included the distant lymph nodes (retroperitoneal and deep inguinal), lung, mediastinum, liver, adrenal glands, and soft tissues.
Secondary Endpoints
The proportion of avoidable local treatments was assessed by considering the number of patients who underwent surgery in each study arm. An unnecessary lymphadenectomy was defined as an extended or superextended lymphadenectomy in the case of pathologically confirmed absence of lymph node involvement (pN0) or stage IV disease.
The onset of side effects was examined in patients who were injected with [18F]F-choline. Local tolerance to injection was examined, and cardiac and respiratory rates, as well as arterial blood pressure, were monitored before and after the PET examination. Finally, any symptoms experienced by patients were recorded both at the nuclear medicine department and through a telephone interview within 24 h after the examination. Toxicity was scored as grade 1 (mild adverse event), grade 2 (moderate adverse event), grade 3 (severe adverse event), grade 4 (life-threatening or disabling adverse event), or grade 5 (adverse event–related death), on the basis of the Common Terminology Criteria for Adverse Events.
RFS was defined as biochemical-recurrence–free survival and radiologic-evidence–free survival. Biochemical-recurrence–free survival was defined as the interval from randomization to the onset of biochemical recurrence (12,13). Radiologic-evidence–free survival was defined as the interval between randomization and the appearance of a PCa recurrence at any radiologic examination (e.g., ceCT, [18F]F-choline PET/CT, or MRI). Finally, OS was defined as the interval between randomization and all-cause mortality.
Statistical Analysis
Eligible patients were randomized with a ratio of 1:1 to one of the two arms for their diagnostic assessment. Each arm included 195 patients, for a total of 390 patients involved in the study. To evaluate the dimension of the simple population, it was assumed that sensitivity for identifying lymph node metastases in this setting of patients was 18% (11). To demonstrate an absolute increase of 20% in the ability of [18F]F-choline PET/CT to identify lymph node metastases, with a significance level (α) of 5% and a power of test of 80%, 137 positive lymph node patients were necessary (2-tailed χ2 test). If a 35% prevalence of lymph node metastases was supposed, 195 patients for each arm were necessary, for a total of 390 patients. The study arms were compared using the χ2 test. The results were expressed as percentages with 95% CIs. For the primary endpoint, a conventional methodology was used to evaluate diagnostic performance in terms of sensitivity, specificity, and accuracy for nodal and distant metastatic groups, together and separately. For secondary outcomes, the proportion of patients with a management effect were compared using the Fisher exact test. Safety profiles were compared using the Student unpaired t test. Finally, survival analysis was calculated using Kaplan–Meier analysis and log-rank testing.
RESULTS
Patient Population
From February 2016 to December 2020, 256 patients were randomly assigned at 7 sites. Table 1 includes the characteristics of patients for each group. Although the number of patients expected was 390, the accrual was stopped. However, the increase in diagnostic performance by [18F]F-choline PET/CT was enriched, as reported below.
Characteristics of Population
In total, 236 patients were considered for the final assessment: 117 in the control arm and 119 in the experimental arm (Fig. 1). Baseline characteristics were similar in each group. Most of the patients had Gleason scores of either 7 or 8, and roughly 65% of the patients had high-risk PCa. Most patients had the T2b stage (36.8% in the experimental arm and 29.2% in the control arm). The proportion of patients with a stage higher than T2 was 13.3% and 12.4% in the experimental and control groups, respectively. In the control arm versus the experimental arm, 41 (35%) versus 46 (38.7%) and 76 (65%) versus 73 (61.3%) patients had intermediate- and high-risk PCa, respectively.
Trial profile.
Diagnostic Accuracy
The detection of lymph node metastases on ceCT was higher in the experimental group (n = 18, 15.1%) than in the control group (n = 9, 7.7%). Additionally, the incidence of bone or distant lymph node or liver metastases on ceCT was slightly higher in the experimental group (n = 12, 10.1%) than in the control group (n = 11, 9.4%). At bone scintigraphy, the experimental group had a slightly higher percentage of positive findings for bone metastases (n = 27, 22.9%) than did the control group (n = 24, 21.1%). PET/CT was positive for local lymph nodes and distant metastases (i.e., distant lymph nodes, bone, lung, or liver) in 41 (35%) and 13 (11%) patients, respectively. In the experimental arm, agreement between ceCT and [18F]F-choline PET/CT for detection of local lymph nodes was 77.8% (n = 91/117).
A histologic reference standard was assessable in 159 patients (n = 73, 62.9%, and n = 86, 72.9%, in the control and experimental arms, respectively). Serial PSA levels within 6 mo from radiotherapy, hormonal therapy, chemotherapy, or a combination of more than one therapy was considered the gold standard in the remaining 77 patients. The most frequently performed surgical procedure was robot-assisted radical prostatectomy (49.3% in the control group and 41.2% in the experimental group), followed by open radical prostatectomy (45.1% in the control group and 48.2% in the experimental group) and laparoscopic radical prostatectomy (5.6% in the control group and 10.6% in the experimental group).
Lymphadenectomy was performed on 69 and 83 patients in the control and experimental groups, respectively. In the control arm, 62 (89.9%) patients were classified as pN0, whereas 7 (10.1%) patients had lymph node metastasis (pN1). Conversely, in the experimental group, 64 (77.1%) patients were pN0 and 18 (21.7%) were pN1. A median of 15 (range, 2–48) and 14 (range, 2–53) lymph nodes was removed in the control and experimental arms, respectively. On the basis of the combination of lymphadenectomy and serial PSA evaluation during follow-up, 66 patients were considered pN1: 34 (29.1%) in the control arm and 32 (27.6%) in the experimental arm. Table 2 presents the diagnostic performance of imaging techniques (ceCT alone vs. [18F]F-choline PET/CT) for detecting lymph node metastases in both arms, with the final pathology finding of the lymphadenectomy serving as the reference standard. On the other hand, Table 3 reports the diagnostic performances for lymph node metastases in both arms by considering the final pathology finding of the lymphadenectomy and serial PSA evaluations as the standard of reference.
Diagnostic Performance for Lymph Node Disease in Control and Experimental Arms, Based on Histopathologic Data
Diagnostic Performance for Lymph Node Disease in Control and Experimental Arms, Based on Histopathologic Data and Serial PSA Levels During Follow-up
On the whole, sensitivities were higher in the experimental arm than in the control one (77.78% vs. 28.57% [P = 0.0661], and 65.62% vs. 17.65% [P = 0.0002], respectively), both in the case of lymphadenectomy as the standard of reference and in the case of histopathology plus serial PSA evaluation as the standard of reference. However, a slight decrease in specificity was reported in both cases (P < 0.02). Possibly, some lymph nodes showing signs of inflammation were considered positive during the analysis of the PET images. Nevertheless, although not statistically significant, the negative predictive values were higher in the experimental arms, independently of disease prevalence.
Tables 4 and 5 describe the diagnostic accuracies of imaging techniques in the different risk groups of patients. Because of the limited number of intermediate-risk patients with positive lymph nodes at histopathology, data were missing. However, in the high-risk group, [18F]F-choline PET/CT was more sensitive than ceCT in detecting pathologic lymph nodes (75% vs. 16.67%, respectively). When the combined reference standard was used, the higher value for the sensitivity in the experimental group than in the control one was confirmed in patients with high-risk PCa. Moreover, in intermediate-risk PCa patients, [18F]F-choline PET/CT registered a sensitivity of 50% and a negative predictive value of 87.88%.
Diagnostic Performance for Lymph Node Disease in Control and Experimental Arms, Based on Histopathologic Data
Diagnostic Performance for Lymph Node Disease in Control and Experimental Arms, Based on Histopathologic Data and Serial PSA Levels During Follow-up
Unnecessary Lymphadenectomy
On the basis of the definition of unnecessary lymphadenectomy, 20 of 117 (17.1%) and 13 of 119 (10.9%) patients received an extended or superextended lymphadenectomy in the case of pathologically confirmed absence of lymph node involvement (pN0) or stage IV disease, respectively, in the control and experimental arms.
Tolerance of [18F]F-Choline Injection
Data about the tolerance of [18F]F-choline injection were available for 63 of 119 (53%) patients. The radiopharmaceutical was well tolerated. No side effects or adverse effects were reported by the patients during the 24 h after the examination (Supplemental Fig. 2).
Survival Analysis
Median follow-up time was 4.43 y (interquartile range, 2.74–5.18 y). At survival analysis, 29 of 117 (24.8%) and 32 of 119 (26.9%) patients in the control and experimental arms experienced recurrence of disease (either biochemical or radiologic recurrence). However, 8 of 117 and 8 of 119 died in the control and experimental arms, respectively, whether related to the cancer or not. Figure 2 reports the Kaplan–Meier analysis for OS and RFS. The 5-y OS was 88.5% versus 92.4% (log-rank test, P = 0.762), and the 5-y RFS was 66.4% versus 67.4% (log-rank test, P = 0.915), in the control and experimental arms, respectively.
Kaplan–Meier curves for OS (A) and RFS (B) stratified according to randomization arm.
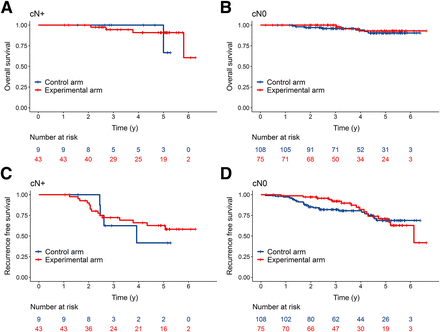
Furthermore, across all patient populations, the detection of positive nodes by histopathology plus serial PSA evaluation correlated with a 5-y OS of 66.7% in the control arm and 90.9% in the experimental arm, as well as a 5-y RFS of 41.7% in the control arm and 62.7% in the experimental arm. However, these differences were not statistically significant (log-rank test, P = 0.6224 for OS and P = 0.6221 for RFS in the control and experimental arms, respectively) (Figs. 3A and 3C). In contrast, among patients with negative lymph node status at histopathology plus serial PSA evaluation, both 5-y OS and 5-y RFS were comparable between the control and experimental arms, with rates of 90.4% versus 93.0% (log-rank test, P = 0.4878) for OS and 68.8% versus 71.0% (log-rank test, P = 0.6160), respectively, for RFS (Figs. 3B and 3D).
Kaplan–Meier curves for OS (A and B) and RFS (C and D) stratified according to positive (A and C) or negative (B and D) lymph nodes at histopathologic and PSA evaluation.
DISCUSSION
In the present prospective study, we found that in lymph node and bone metastases, [18F]F-choline PET/CT had a per-patient diagnostic accuracy superior to that of conventional imaging alone in men with intermediate- and high-risk PCa. This finding reinforces data from retrospective single-center studies that have suggested potentially higher accuracy for [18F]F-choline PET/CT than for ceCT and bone scanning in the staging of disease (7,14–16). Beheshti et al. (17) correlated histopathologic findings after radical prostatectomy and extended lymphadenectomy in 111 patients with intermediate- and high- risk of PCa. Using a patient-based analysis, they reported a sensitivity of 45% and a specificity of 96% for lymph node disease. The authors underlined the failure of [18F]F-choline PET/CT to detect the presence of micrometastatic lymph nodes. Poulsen et al. (18) reported, at patient-based analysis, a slightly higher sensitivity of 73.2% and a quite similar specificity of 87.6%. Evangelista et al. (7), by a comparison between conventional imaging (i.e., ceCT) and [18F]F-choline PET/CT, demonstrated that the latter is more accurate for the identification of lymph node and distant metastases than are conventional modalities (46.2% vs. 69.2% for CT and PET/CT, respectively). Moreover, [18F]F-choline PET/CT is able to detect bone marrow metastases early, with sensitivity of 79%–100% and specificity of 77.2%–97% (7,19). In accordance with these latter 2 authors, the present study demonstrated [18F]F-choline PET/CT to be superior to ceCT alone for the identification of lymph node disease, with sensitivities of 78% and 66%, respectively, as compared with histopathologic evaluation or the combination of histopathology and serial PSA levels. However, early detection of lymph node disease before surgery can be valuable in reducing the number of aggressive lymphadenectomy procedures or guiding selective lymphadenectomy to avoid unnecessary complications. In the present study, we found about an 8% reduction in unnecessary extended lymphadenectomy with [18F]F-choline PET/CT as compared with ceCT—an interesting finding considering the high incidence of PCa and the complications due to the surgical procedure. However, much effort is ongoing to identify algorithms able to reduce the number of unnecessary extended lymphadenectomy procedures, using PSMA-based PET (20).
After radical prostatectomy, approximately 35% of patients will experience biochemical recurrence within 10 y (21–24). One reason for this high rate of treatment failure is the limited accuracy of conventional imaging in detecting metastatic disease ab initio. However, to our knowledge, this was the first prospective study that evaluated the effect on the outcome of [18F]F-choline PET/CT versus conventional imaging. Indeed, the 5-y OS was slightly higher in the experimental arm than in the control arm (92.4% vs. 88.5%, respectively), although not statistically significant. However, if the incidence rate range provided by Giona were used (6.3–83.4 per 100,000 people) (25), the numbers of individuals affected by intermediate- and high-risk PCa would range from 6.3 to 83,400. Subsequently, approximately 6,552–86,736 individuals could benefit from a 4% increase in survival.
On the other hand, 5-y RFS was similar in both groups. Recently, a study by Urso et al. (26) demonstrated that, in real-world experience, [18F]F-choline PET/CT as a tool for the initial management of PCa had a relevant impact in terms of therapy selection and was associated with longer biochemical RFS. Preliminary data about the prognostic value of [68Ga]Ga-PSMA-11 PET–detected nodal involvement in 251 patients without distant metastases, after a follow-up period of 54 mo, were discussed at the last European Congress of Nuclear Medicine (27). Patients without lymph node involvement on [68Ga]Ga-PSMA-11 PET imaging were free from treatment failure longer than those with N1M0 disease (hazard ratio, 2.1; range, 1.2–3.17; P < 0.01). Conversely, CT- and bone scan–defined N0M0 versus N1M0 were not prognostic (hazard ratio, 0.6; range, 0.1–2.4; P = 0.45). In this trial, we confirmed that the presence of positive nodes at histopathologic/PSA evaluation impacted long-term survival outcomes; additionally, [18F]F-choline PET/CT further stratified them, although the observed differences in OS and RFS between the control and experimental arms did not reach statistical significance. Interestingly, among patients with negative lymph node status, there were no notable differences in 5-y OS and RFS rates between the 2 arms. A prolonged follow-up is undoubtedly necessary to comprehensively evaluate the impact of [18F]F-choline PET on long-term outcomes for patients with intermediate- and high-risk PCa.
The current trial had some limitations. First, the inability to mask the imaging modality during randomization introduced potential bias. Second, histopathologic assessment was not available in some participants, especially those with local or distant nodal metastases who underwent radiotherapy. However, to overpass this limitation, we used a reference standard incorporating 6 mo of follow-up with serial PSA evaluations and repeated imaging, if needed, which is considered a robust method. Finally, although there has been a large expansion of PSMA-targeted tracers, these agents remain unavailable in certain countries and smaller hospitals. Therefore, the availability of alternative radiopharmaceutical agents for PET imaging could prove invaluable in furthering the expansion of nuclear medicine modalities, particularly in underserved regions and smaller health care facilities.
CONCLUSION
[18F]F-choline PET/CT has demonstrated higher diagnostic performance than conventional imaging in detecting both lymph node and bone metastases. Moreover, it can reduce the rate of unnecessary extensive lymphadenectomy in more than 8% of patients. Finally, it can slightly affect the 5-y OS, increasing the survival rate to 4%. These results provide additional evidence supporting the discontinuation of conventional imaging for staging PCa.
DISCLOSURE
This clinical trial was supported for a limited number of patients by IASON GmBh (18F-choline supply for 73 patients and a small amount of the insurance payment for patients). IASON GmBh had no role in the study design, data collection, data analysis, data interpretation, or writing of the paper. Prof. Giacomo Novara and Prof. Filiberto Zattoni have additionally supported the clinical trial by a grant from the University of Padua (for the supply of 18F-choline in the remaining enrolled patients and other additional costs). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is PET/CT with [18F]F-choline able to identify lymph node and systemic involvement during initial staging in intermediate- and high-risk PCa patients?
PERTINENT FINDINGS: A prospective controlled, open, randomized multicenter phase III trial involving 7 Italian centers enrolled 236 patients (117 in the control arm and 119 in the experimental arm). In the experimental arm, the sensitivity for lymph node metastases was higher than in the control arm (77.78% vs. 28.57% and 65.62% vs. 17.65%, respectively).
IMPLICATIONS FOR PATIENT CARE: In the initial staging of PCa, [18F]F-choline PET/CT exhibited superior diagnostic performance to conventional imaging for detecting metastases, thus supporting the consideration of discontinuing conventional imaging in this setting of disease.
ACKNOWLEDGMENTS
The study group coinvestigators are Luigi Corti, Matteo Sepulcri, Vittorina Zagonel, Umberto Basso, Marco Maruzzo, Luca De Zorzi, Tommaso Silvestri, Enrico Pizzirani, Filiberto Zattoni, Giuseppe Costa, Andrea Guttilla, Andrea Agostini, Angelo Porreca, Paolo Corsi, Massimo Dal Bianco, Mario Gardi, Giovanni Maria Ceresoli, Fabio Matrone, and Roberto Bortolus. We thank all the staff at the Clinical Trials and Biostatistics Unit of the Veneto Institute of Oncology for trial coordination, including Dr. Marina Salomoni (clinical research coordinator) and Drs. Marco Braggion and Beatrice Barbaro (monitoring study coordinators).
Footnotes
Published online Jun. 6, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 3, 2024.
- Accepted for publication May 7, 2024.