Abstract
The early history of the use of radioactive iodine (RAI) is complicated and interesting, and also difficult to discover, especially since several histories have presented inaccurate content. This article is a comprehensive review of the accomplishments of Saul Hertz. Extensive use of primary-source verification has clarified several issues, including the question of whether Hertz alone conceived and asked the pivotal question: “Could iodine be made radioactive artificially?”; on what date RAI was first used to treat hyperthyroidism; and why 2 articles on the first use of RAI for treatment of hyperthyroidism, from 2 different sets of authors from the same department of the same institution, appeared adjacent to each other in the same issue of the Journal of the American Medical Association in 1946. Our review also chronicles several major challenges that Hertz overcame to produce his pivotal work. Hertz was clearly the originator and a visionary of RAI therapy in benign and malignant thyroid disease. We believe he can be considered one of the fathers of nuclear medicine. Hertz’s paradigm-changing work was a pivotal medical discovery of the 20th century. The legacy of Hertz continues while the application of RAI therapy continues to evolve. RAI therapy remains the preferred treatment in most situations for autonomous nodules and toxic multinodular goiter and remains a safe and effective treatment for Graves disease after more than 80 y of global clinical use. RAI treatment of differentiated thyroid cancer remains a first-line treatment for most patients after surgery, especially for those with intermediate- or high-risk disease.
In the landscape of medical history and innovation, few figures exemplify the blend of groundbreaking scientific achievement and selfless service as well as Saul Hertz does. Born on April 20, 1905, Hertz’s journey in medicine began with his graduation from Harvard Medical School in 1929, a time of strict quotas for outsiders. He served as chief of the Thyroid Unit at Massachusetts General Hospital (MGH) from 1931 to 1943. Despite the promise of a burgeoning research and clinical career, Hertz’s path took a detour as he answered the call of duty, serving as a commander in the U.S. Navy during the tumultuous years of World War II from 1943 to 1945. Tragically, his life and career were cut short when he passed away on July 28, 1950, at the age of 45 because of a myocardial infarction, as confirmed by autopsy. This review article seeks to explore not just the professional milestones of Hertz but also the personal and historical context that shaped his remarkable, albeit too brief, journey through life and medicine (Table 1).
Accomplishments of Saul Hertz
Four scientific advancements and discoveries provide the basis for the pivotal question and subsequent research and clinical work of Hertz. These include the understanding of the role of iodine in thyroid metabolism and function, the discovery of radioactivity, the development of the tracer principle, and the production of artificial radioactivity (1).
On November 12, 1936, Karl Compton, president of the Massachusetts Institute of Technology, presented to the faculty at the Harvard Medical School a guest lecture entitled, “What Physics Can Do for Biology and Medicine” as part of a weekly lecture series and luncheon. At the conclusion of the lecture, Hertz solely conceived and spontaneously asked the pivotal question “Could iodine be made radioactive artificially?” Karl Compton was uncertain and said he would look into it. He wrote back to Hertz on December 15, 1936, apologized for the delayed response, and wrote, “Iodine can be made artificially radioactive.” In fact, Enrico Fermi had produced 128I in 1934, and earlier in 1934, Frederic Joliot and Irene Joliot-Curie created artificial radioactivity, for which they received the Nobel Prize for Chemistry in 1935. Letters between Hertz and Compton make it clear that the idea of using radioactive isotopes to study metabolism came solely from Hertz (1–4). The fact that Hertz solely asked the question was confirmed by James Means, chief of medical services at MGH, in a letter to the Markle Foundation (Fig. 3 in (2)) in which he stated, “when it became apparent that there might be radioactive isotopes of iodine, it at once occurred to Hertz that we might make use of them to solve a problem we were already working on” (1–5). Robley Evans, director of the Massachusetts Institute of Technology Radiation Laboratory, also wrote a letter of recommendation to the Navy confirming that Hertz was the one who conceived the idea of using radioactive iodine (RAI) for research and therapy of thyroid disease (5).
Hertz collaborated with Arthur Roberts, a medical physicist who was hired at Massachusetts Institute of Technology by Robley Evans. Earle Chapman was an endocrinologist in the MGH Thyroid Clinic. When Hertz was leaving MGH to join the Navy in 1943, Hertz asked Chapman to supervise the clinical trials and follow the established protocols, to which Chapman agreed. However, Chapman changed the protocols. John Stanbury was an endocrinologist in the Thyroid Clinic, who wrote a book on the history of the MGH Thyroid Clinic from 1913 to 1990. Unfortunately, the book contained significant misinformation. Roberts wrote a letter to Stanbury on April 3, 1991, in an attempt to correct the misinformation. Roberts also stated in the letter, “Evans made it a condition of my employment that his name was to appear on all publications” (2). Hertz and Roberts included Evans on the first 2 papers, but not after that, since Evans never participated in any of the studies (5). It is to be noted that Hertz and Roberts designed the research, executed the work, analyzed the data, and wrote the papers (4). Evans was not involved in any of those activities. Evans directed the laboratory that initially provided the neutron source to produce RAI. The Massachusetts Institute of Technology cyclotron produced greater quantities of RAI that were critically important for RAI therapy.
Although it is often assumed science and scientific endeavor occur in a world isolated from the prejudices and mores of the time, this is sadly hardly ever the case. At the time Hertz was working and publishing his research, he was living and working in a city in which antisemitic opinion and actions had become commonplace. In the 1930s, Boston was the most anti-Jewish city in the United States and the headquarters of an organization called the Christian Front (6). Until 1941, this organization, at the very time that Hertz was working in Boston, was financed and led by the national socialist (Nazi) government in Germany. Even after the United States entered the war against Germany, the organization continued to flourish and organize antisemitic protest marches through the streets of Boston. The organization was implicated in physical attacks on Jewish men and boys up to the end of 1943.
Hertz faced and overcame several major challenges during his career: pushback from surgeons, who were concerned that he was taking business from them; interruption of his clinical studies by World War II; questionable ethics in medical publishing (ghost authoring); others trying to steal credit for his work; and confrontation with strict quotas and restrictions—he was a target of personal and systemic antisemitism.
The true story of Hertz includes elements of exploitation, failure to honor those who served in the war, greed, stolen intellectual property, and antisemitism. Evans deliberately muddied the waters as to the truth regarding whether Hertz was the one who asked the pivotal question. The truth was explained in April 2016 by MGH’s chairman emeritus of the Department of Radiology, James Thrall, who stated, “…Chapman and Evans had basically stolen Hertz’s work…the most flagrant, unethical, academically reprehensible behavior…worst yet…. Chapman and Evans spent a great deal of time and effort rewriting history” (7).
In 1946, there were 2 adjacent publications in the same May 11 issue of Journal of the American Medical Association, from the same laboratory on the same topic—the first successful use of RAI to treat hyperthyroidism. The editor of that journal from 1924 to 1950 was Morris Fishbein, who was aware of the previous 6 papers by Hertz and Roberts. He first received a paper from Chapman and Evans. Fishbein sent it back for revision, since it was too long. Fishbein was also concerned that the studies on the same topic from the same laboratory did not include Hertz and Roberts and did not even reference them. Fishbein contacted Means, who assured Fishbein that the Chapman and Evans article was legitimate and that their article was based on a series of different patients. Fishbein contacted Hertz and asked him to submit his paper with the results of his first clinical trial, which he did. After negotiation, Fishbein published both papers next to each other and placed the Hertz and Roberts paper first, to acknowledge their pioneer status, since those studies were done first (8,9). This issue is discussed further in a paper by Obaldo and Hertz (5).
History records Hertz, with Roberts, as a pioneer of RAI therapy and as establishing nuclear medicine as a new specialty of medicine (Figs. 1 and 2). Hertz created a dynamic and enduring fundamental change in modern medicine. He influenced the convergence of the sciences and collaborative teams as well as the use of uptake testing and dosimetry to develop precision targeted medicine. His legacy lives on today (Tables 2 and 3).
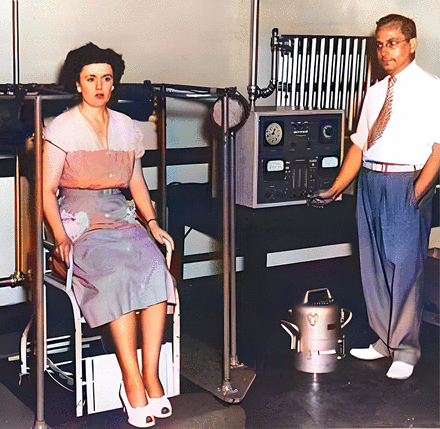
Hertz with his assistant and multiscaler probe. (Colorized version made using hotpot.ai deep learning.)
Boston Traveler newspaper of September 30, 1949, announcing new research division where radioactive isotopes were to be used in study and treatment of disease.
Awards and Honors Received by Saul Hertz
Legacy of Saul Hertz
We also acknowledge John Lawrence for use of 32P for treatment of leukemia in 1936 and polycythemia vera in 1937. At the Lawrence Berkeley National laboratory, Lawrence’s team was also investigating RAI in the late 1930s and early 1940s and began treating hyperthyroid patients in October 1941 (1). Also acknowledged is Charles Pecher for treatment of bone sarcoma with 89Sr in 1941. Today, we stand on the shoulders of these giants as we continue our efforts to develop next-generation theranostics.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
We acknowledge Barbara Hertz, who provided the 2 figures and primary source material for this article.
Footnotes
Published online Mar. 7, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 26, 2024.
- Revision received January 30, 2024.