Abstract
P911
Introduction: The global obesity epidemic poses a major challenge for health care systems worldwide. Thus the search for interventions to achieve sustainable weight loss reaches high priority including a thorough investigation of principle biological and behavioral mechanisms in individuals with obesity (OB). As a key biological mechanism in OB and putative pharmacological treatment target, the brain cholinergic system gains interest since cholinergic modulation of brain reward and attentional networks seems to play a crucial role in information processing about salience. The aim of this study is to further investigate changes in α4β2* nicotinic acetylcholine receptors (nAChR) in response to salient, high-caloric food cues together with assessments of changes in large-scale networks and behavioral ratings in individuals with OB and normal-weight controls (NW).
Methods: Thirty-one individuals with OB (n=15; 10♀; age 38±14 yrs; body-mass-index, BMI, 38±3 kg/m2) and NW (n=16; 13♀; 27±7 yrs; BMI 22±2 kg/m2) underwent simultaneous PET-MRI with (-)-[18F]flubatine twice on separate days (one resting-state examination and one under food cue stimulation) using a bolus-infusion protocol (294±7 MBq) with list-mode acquisition 0-60 min and 120-165 min p.i. paralleled by anatomical MP-RAGE and functional echo-planar imaging sequences. Total distribution volumes VT were estimated as the ratio between mean (-)-[18F]flubatine activity in tissue between 120 and 165 min and free parent (-)-[18F]flubatine in plasma obtained from venous blood. Food pictures were shown 120-135 min p.i.. During scanning, a visual analogue scale (VAS) to assess states of hunger/satiety, appetite, disinhibition, craving, and taste. Eating behavior was measured using the Three-Factor Eating Questionnaire (TFEQ).
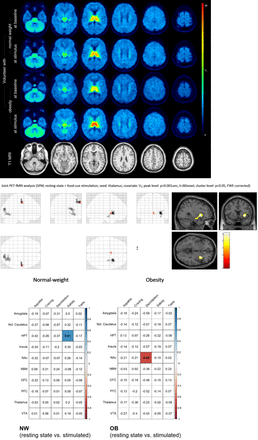
Results: Resting-state VT data showed no significant difference between OB and NW. Under stimulation, VT was higher in OB compared with NW in the thalamus (28.8±2.1 vs. 25.1±1.9, p=0.03), particularly in those with high TFEQ (30.7±4.5 vs. 25.1±3.3, p=0.01). Intra-individually, VT values in NW did not changed between resting-state and stimulus condition (in the thalamus 26.0±1.6 vs. 25.7±1.5, p=0.9; ventral tegmental area, VTA, 17.5±1.2 vs. 17.5±1.3, p=1.0; nucleus basalis of Meynert, NMB, 11.7±1.0 vs. 11.2±0.9, p=0.5) while in OB tendencies toward higher values under food cue stimulation compared with resting state were registered (in the thalamus 26.7±1.8 vs. 28.4±2.0, p=0.15; VTA 15.9±1.4 vs. 16.7±1.5, p=0.11; NMB 10.0±1.1 vs. 10.4±1.1, p=0.24). Seed-based analysis on functional connectivity using the VT of the thalamus as a covariate in comparison of resting-state versus stimulation (p<0.05; FWE-corrected) showed strenghtened connectivity with parieto-occipital and central regions of the brain in NW, i. e. primarily the dorsal attentional network, while there was a strengthened connectivity between the thalamus and the insula, primarily the salience network, in OB. Finally, correlation analyses between VT with different behavioral measures (VAS) showed significant correlation between VT in the hypothalamus and measure for satiety in NW (r=0.61, p<0.01) while in OB significant correlation with measures of disinhibition and the nucleus accumbens was found (r=-0.65, p<0.05).
Conclusions: Compared with NW, individuals with OB showed altered α4β2 nAChR availability, particularly in the thalamus, the mesoaccumbens system and in the basal forebrain in response to food cue stimulation. These changes are associated with an attentional bias towards incentive food cues and likely changes in sensory perception depending on α4β2 nAChR availability. Taken behavioral measures into account, these data might be a further hint for disturbed hedonic pathways with reduced top-down control of mesolimbic reward centers in OB mediated by α4β2 nAChR availability and probably represent a model of higher thalamic output, higher distractibility towards rewarding food cues.