Abstract
P698
Introduction: To induce damage by Auger electrons that leads to cell death, targeting DNA should be a highly efficient strategy. Auger electron-emitting 191Pt is a promising candidate for DNA targeting because platinum itself binds to DNA directly. Here, we developed 191Pt-labeled agents targeting the amplified oncogene MYCN of DNA in neuroblastoma to explore the therapeutic potential of Auger electrons. Additionally, the design of 191Pt-labeled agents for in vivo use was investigated in mice by labeling common tumor-targeting ligandswith191Pt.
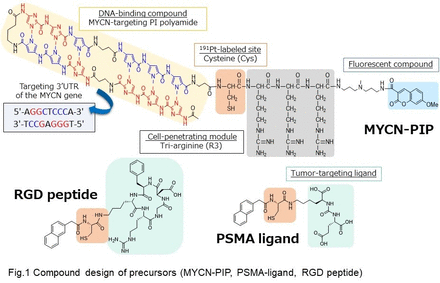
Methods: We developed the MYCN gene-targeting PI polyamide (MYCN-PIP) compound labeled with n.c.a. 191Pt as an example compound (Fig. 1). Its targeting and DNA damaging/cytotoxicity properties were evaluated in cells using the gel electrophoretic mobility shift assay (ESMA), a cellular uptake assay, a DNA-binding assay, a cytotoxicity assay, gene expression analysis, fluorescence in situ hybridization (FISH) analysis, and immunostaining. Additionally, regarding in vivo characteristics of the 191Pt-labeled agents, the 191Pt-labeled PSMA-targeting ligand and integrin-targeting cyclic RGD peptide (Fig. 1) were evaluated with their biodistribution investigated in mice bearing tumor xenografts.
Results: 191Pt-MYCN-PIP was successfully synthesized with a radiochemical purity of >99%. The ESMA suggested that 191Pt-MYCN-PIP bound to the target genome sequence of the MYCN gene. In vitro assays showed that 191Pt-MYCN-PIP bound to DNA efficiently (~40%ID/mg genomic DNA) and caused DNA damage, decreasing MYCN gene expression, MYCN signaling in FISH analysis, and cell viability, especially in MYCN-amplified Kelly cells (Fig. 2). The biodistribution of the 191Pt-labeled tumor-targeting ligands revealed that the specificity of the ligands was maintained in vivo to a certain degree; target-positive tumors had 3-5 times higher uptake than negative tumors. However, the 191Pt activity reacted with and bound to blood proteins like serum albumin containing Cys residues rapidly after intravenous injection.
Conclusions: The in vitro properties of 191Pt-MYCN-PIP showed the potential for targeting key oncogenes in cell survival to bring a therapeutic efficacy for Auger electron-emitting drugs. For future work with 191Pt-labeling drugs targeting both DNA and tumors, suitable multidentate ligands for 191Pt labeling need to be developed in order to maintain the DNA-binding ability of 191Pt after tumor uptake and to keep 191Pt-labeled intact in the blood with low reactivity to serum proteins.