Abstract
P685
Introduction: Iomab-B (131I-apamistamab), a radioimmunoconjugate to CD45, delivers high-dose targeted radiation to hematopoietic cells allowing for myeloablation and eradication of leukemic cells, enabling patients with active R/R AML to proceed to allogeneic hematopoietic cell transplant (HCT). The safety and efficacy of this targeted high-dose radiation delivery as an induction and conditioning regimen was investigated in the SIERRA randomized controlled Phase 3 study comparing the rate of durable complete remission (dCR) at 6 months between Iomab-B based conditioning followed by HCT vs. physician’s choice of conventional care (CC).
Methods: Patients 55 years or older with active R/R AML were randomized (1:1) to Iomab-B or CC arms. Patients on Iomab-B arm received Iomab-B with fludarabine and total body irradiation (2 Gy) followed by HCT. Pts on CC arm received physician’s choice of salvage therapy. Patients on CC arm achieving CR received physician’s choice conditioning and HCT, and those that did not were eligible to cross over to the Iomab-B arm. Patients received individualized doses of Iomab-B based on evaluation of organ-specific uptake and dosimetry estimates from gamma camera imaging following tracer dose administration. The estimated total integrated activity (TIA) and organ-specific absorbed dose estimates were calculated for each patient and the prescribed activity dose of Iomab-B was determined individually by limiting the estimated absorbed dose to liver to 24 Gy.
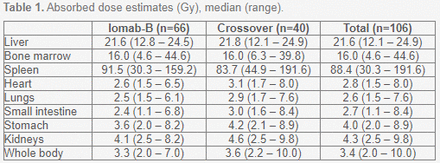
Results: 153 patients were enrolled in the SIERRA trial, 76 in the Iomab-B arm and 77 in the conventional care arm. All patients who received the therapeutic dose of Iomab-B (n=66) underwent HCT vs. 14 (18.2%) on the CC arm, with 44 CC arm patients crossing over to the Iomab-B arm and 40 receiving the therapeutic dose. The primary endpoint of dCR at 6 months strongly favored Iomab-B with 22% dCR vs. 0% for CC (p<0.0001) and event-free survival (EFS) at 6 months was 26% vs. 0.2% for Iomab-B vs. CC (p<0.0001). Iomab-B followed by HCT was well tolerated with lower rates of sepsis for Iomab-B vs. CC (6.1% vs. 28.6%), and respectively 43.9% vs. 50% febrile neutropenia and 15.2% vs. 21.4% mucositis. The median (inter-quartile range) effective half-life of Iomab-B clearance for patients receiving the therapeutic dose was 34.3h (27.3h-40.7h) for liver, 42.0h (37.3h-50.4h) for bone marrow (estimated using sacral imaging), 35.9h (29.7h-45.4h) for spleen, and 42.5h (39.2h-51.3h) for whole body, with corresponding time-activity curves illustrated in Figure 1 and resulting organ-specific absorbed dose estimates given in Table 1.
Table 1. Absorbed dose estimates (Gy), median (range).
Conclusions: Iomab-B based conditioning followed by HCT resulted in statistically significant improvement in dCR at 6 months and a favorable safety profile. Treatment with Iomab-B was able to safely deliver high doses of targeted radiation to leukemic cells, highlighted by the doses to spleen and bone marrow, with successful marrow engraftment, far outweighing what would be safely deliverable using total body irradiation.