Abstract
P67
Introduction: Deep learning approaches for attenuation correction (AC) of bone components on chest positron emission tomography–magnetic resonance images (PET/MRI) is challenging due to the limited delineation of the bone with low proton density and the difference in the subject’s body shape between training data sets. In our previous study, we proposed using unsupervised generative adversarial networks (GANs) with adaptive layer-instance normalization for image-to-image translation (U-GAT-IT) in combination with a modality-independent neighborhood descriptor (MIND) to create pseudo-CT (pCT) with bone components from Zero echo-time (ZTE) MRI for AC in the chest. The purpose was to assess the feasibility of using the 2.5-dimensional (2.5D) method for the generation of bone components from ZTE and to compare the quantitative values of generated pCT and reconstructed PET with the 2D method and conventional MRI-based bone-lacking method.
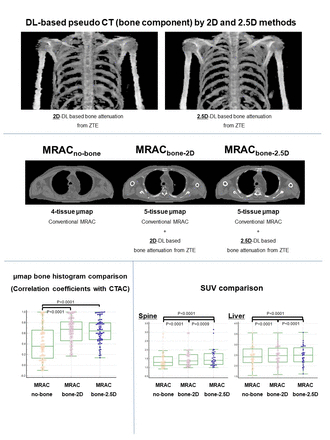
Methods: Three hundred and sixty patients who underwent chest FDG PET/MRI with central-frequency-adjusted ZTE were retrospectively analyzed. Unpaired training data included bias-corrected ZTE and CT components of PET/CT and were utilized for training unsupervised GANs with a U-GAT-IT/MIND model. For the 2.5D method, the value of the loss function with MIND was calculated for each of the three consecutive 2D slices. The weighted sum of the values was then obtained as the result of 2.5D MIND loss function. A pCT-based AC map with bone (MRACbone-2D and MRACbone-2.5D) was created by merging the segmented bone map onto a conventional MRI-based bone-lacking AC map (MRACno-bone) and then applied to PET reconstruction on the offline workstation. Thirty-six cases with ZTE PET/MRI and PET/CT in the same patients were used to compare MRACbone-2D, MRACbone-2.5D, MRACno-bone and CT-based AC map (CTAC) to validate the model for image qualities, CT histograms, and standardized uptake values (SUV) after AC. The image quality of each pCT was evaluated by two board-certified radiologists using a five-point scoring system with respect to bone delineation and bone continuity. Fixed regions of interest (ROI) were placed in the spine and rib on the AC maps for histogram comparison and in the spine and liver on reconstructed PET for mean SUV (SUVmean) comparison. The similarity of the histogram by MRACbone-2D, MRACbone-2.5D, and MRACno-bone to CTAC was assessed by the correlation coefficients. Wilcoxon’s signed rank test was used to compare the correlation coefficients and SUV means between AC maps.
Results: The image-quality scores of MRACbone-2.5D for bone delineation and bone continuity were significantly higher than those of MRACbone-2D (p<0.05). The correlation coefficients of the histogram for MRACbone-2.5D and MRACbone-2D were significantly higher than MRACno-bone in the spine and rib (p<0.0001). The coefficient for MRACbone-2.5D was not significantly different from that for MRACbone-2D (p>0.05). The Spine and liver SUV mean by MRACbone-2.5D and MRACbone-2D were significantly larger than that by MRACno-bone (p<0.0001). Those by MRACbone-2.5D were also significantly larger than that by MRACbone-2D. (p<0.0001).
Conclusions: 2.5D deep-learning (DL)-based AC map generation with bone component was feasible using unpaired PET/MRI and PET/CT datasets. The 2.5D method showed a higher image quality of AC map than the 2D method. AC maps by DL with 2.5D methods yield higher SUV in the bone and liver than those by 2D methods and conventional MRAC.