Abstract
P617
Introduction: Recently, radio-labelled FAP inhibitors (FAPIs) has been widely evaluated in diverse diseases, especially difference malignancies, in malignant assessment, tumor staging and re-staging. The clinical significance on radiotherapy target volume delineation has also been reported. In this study, we made clinical attempt to evaluate the potential usefulness of 99mTc-HFAPi in assessing early response to chemotherapy in lung cancer patients, especially in cases who are clinically suspected of progression disease (PD).
Methods: This is a single-centre prospective diagnostic study (Ethic approved No.: XJTU1AF2021LSK-021 of the First Affiliated Hospital of Xi'an Jiaotong University and ChiCTR2100048093 of the Chinese Clinical Trial Register). Between June 2021 and September 2022, patients with pathologically confirmed lung cancers who just finished cycles chemotherapy underwent 99mTc-HFAPi SPECT/CT within 1 month. Lesion based analyses was conducted with reference simultaneously CT or MRI. When more than one measurable lesion is present, all lesions up to a maximum of five highest uptake (and a maximum of two highest uptake lesions per organ) representative were recorded and measured. Positive uptake was defined as at least one lesion with tumor-to-background (T/B) ratio >3.0 in one patient. Response to chemotherapy was evaluated by using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The patients were then follow-up and changes of oncologic management were recorded. Re-evaluation was performed within 2-4 months after the change of the regimen according to RECIST 1.1.
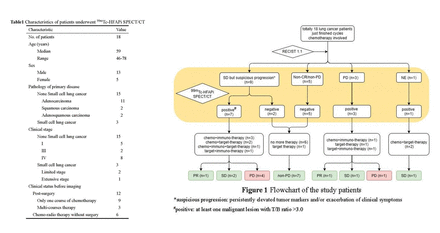
Results: Totally, 18 consecutive patients (13 males; median age 59 years) with 39 malignant lesions were analyzed. Clinical characteristics was summarized in Table 1. After cycles of chemotherapy, 9 of them were assessed as stable disease (SD) but showed s and/or exacerbation of clinical symptoms, 5 were non-complete response/non-progressive disease (Non-CR/non-PD), 3 were PD and 1 was inevaluable (NE). Seven out of the 9 SD cases exhibited positive uptake of 99mTc-HFAPi. Multi-disciplinary treatment (MDT) administered the 7 positive cases a more proactive treatment. 42.86% (3/7) of them benefited from the changing of the regimen and partial response (PR) or SD were reached per RECIST 1.1. Altogether 2 SD and 5 non-CR/non-PD patients showed negative uptake of 99mTc-HFAPi. Six of them received no more treatment and the remaining 1 received oral targeted drug. Subsequent response assessment of them demonstrated a negative predictive value (PPV) of 100% (7/7). The remaining 3 PD and 1 NE patients all showed positive uptake of 99mTc-HFAPi and underwent a next course of treatment. Continuous PD was shown in 1out of the 4 patient and others benefited from the treatment (1 with PR and 2 with SD). The detailed scheme was shown in Figure 1.
Conclusions: In lung cancer patients undergoing chemotherapy with inconclusive SD per the RECIST 1.1, 99mTc-HFAPi SPECT/CT may have a complementary role in assessing tumor activity and detecting suspected disease progression, which assisted clinicians’ additional value in oncological regimen decisions.