Abstract
P533
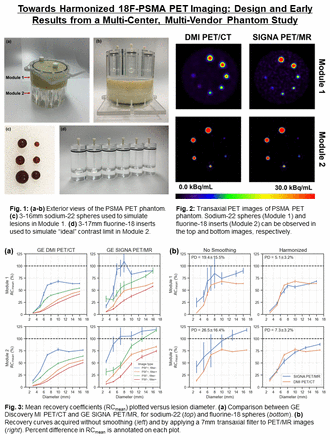
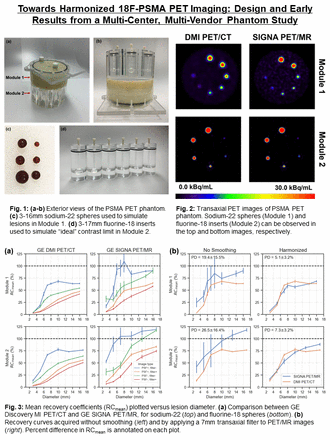
Introduction: The development of positron emission tomography (PET) tracers targeting prostate-specific membrane antigen (PSMA) has allowed for enhanced detection of metastatic prostate cancer (mPCa) as compared to conventional imaging modalities. PSMA PET has greatly improved clinical decision making for oncologists and changed management plans for patients with mPCa. Harmonized PET/CT imaging may enable the development of multi-centre studies and lead to improved predictive modeling. Scanner performance is conventionally validated using the NEMA NU 2 methodology, in which tumors are modeled using 10-37mm fillable spheres. However, this approach neglects to model the smaller (3-10mm) high-contrast metastases that are frequently observed in PSMA PET imaging. We previously developed a portable phantom for PSMA PET quantification (Fedrigo et al., EANM 2021), which uses a combination of 22Na sources (module 1) and 18F inserts (module 2) with sphere sizes designed to characterize this important, yet unexplored contrast recovery space. In the present work, we highlight the design and early results from a multi-center, multi-vendor harmonization study using this phantom. We specifically compare the performance of a PET/CT and PET/MRI scanner, though this work is being expanded to include a network of scanners across North America.
Methods: The phantom was scanned in a GE Discovery MI PET/CT and GE SIGNA PET/MR from different institutions. The phantom was filled with 6 MBq of [18F]FDG and 5x2.5min PET scans were acquired. Data were reconstructed with TOF ordered subsets expectation maximization (TOFOSEM) using site-specific parameters (8 subsets and 4 iterations for PET/CT, 28 subsets and 4 iterations for PET/MR). Point-spread function (PSF) modeling and CT-based attenuation correction (CTAC) was applied. Decay correction was applied to account for 18F radioactive decay. For harmonization, images were smoothed using transaxial Gaussian filters ranging from 1-10mm, followed by a 1/4/1 weighted convolution applied in the axial plane. Spheres were segmented using 41% of SUVmax fixed thresholding (41% FT) and recovery coefficients (RC) were computed from 5 noise realizations of SUVmean. As a measure of harmonization, mean percent difference (PD) was used to compare RCs between the PET/CT and PET/MR systems.
Results: In module 1, RCs were 3.1%, 42.4%, 63.4%, and 63.9% for the DMI PET/CT, as compared to 17.0%, 93.2%, 109.6%, and 90.3% for the SIGNA PET/MR, in the 3mm, 5mm, 8mm, 16mm spheres, respectively. Similarly, RCs were 13.5%, 32.9%, 77.7%, and 76.7% for the DMI PET/CT, compared with 52.7%, 88.8%, 105.4%, 119.0%, for the 3mm, 5mm, 10mm, 17mm spheres, respectively (module 2). Mean percent noise was higher for the DMI when compared to SIGNA with values of 2.1% and 9.2%, respectively (module 1). The same trend was observed for the other component of the phantom, which increased from 1.6% to 8.5%. Before applying post-reconstruction smoothing, PD between the scanners was 19.4±15.5% and 26.5±16.4% in module 1 and module 2, respectively. After applying a 7mm Gaussian filter to the PET/MR, PD was reduced to 5.1±3.2% (module 1) and 7.3±3.2% (module 2).
Conclusions: A multi-center, multi-vendor harmonization study is underway to improve harmonization of 18F-PSMA PET imaging across North America. Overall, the RCs were higher for the GE SIGNA PET/MR scanner, as compared to the DMI PET/CT, which can likely be attributed to the smaller voxel size used in the clinical reconstruction parameters (1.39mm vs. 2.73mm) and additional iterative updates used during reconstruction (i.e. 112 for PET/MR vs. 32 for PET/CT). Based on our results, applying a 7mm Gaussian filter to smooth images obtained with the GE SIGNA PET/MR was needed to achieve harmonization with the DMI PET/CT scanner at BC Cancer. In next steps, this phantom study will be implemented at 10-20 sites across North America, ideally establishing a benchmark to compare and harmonize lesion quantification in PSMA PET imaging.