Abstract
P361
Introduction: Evans blue (EB) moiety provides reversible, medium affinity binding to albumin in vivo. The purpose of this study is to optimize EB modified prostate-specific membrane antigen (PSMA) ligands, that could maximize the absolute tumor uptake and therapeutic efficacy of prostate cancer.
Methods: The lead compound DOTA-PSMA-EB-01 (denoted as LNC1003) was synthesized based on PSMA-targeting agent and radiolabeled with 177Lu and 225Ac for radioligand therapy. Binding affinity and PSMA targeting specificity were verified through cell uptake and competition binding assay. SPECT/CT imaging of 177Lu-LNC1003 was performed to visualize the radioligand distribution in vivo. Biodistribution studies of 177Lu/225Ac-LNC1003 in both 22RV1 and PC3-PIP tumor bearing mice were performed to confirm the absolute tumor uptake. Radioligand therapy studies were conducted to evaluate the therapeutic efficacy of 177Lu/225Ac-LNC1003.
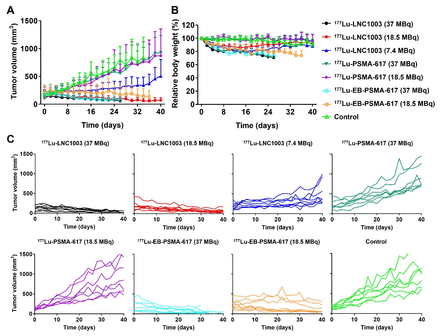
Results: High binding affinity of LNC1003 (IC50 = 10.77 nM) for PSMA was confirmed in vitro. SPECT imaging of 177Lu-LNC1003 demonstrated significantly improved tumor uptake and retention than those of our previously reported 177Lu-EB-PSMA and FDA approved 177Lu-PSMA-617. Biodistribution studies of 22RV1 tumor mice with moderate level of PSMA further confirmed the remarkably higher tumor uptake of 177Lu-LNC1003 (138.87 ± 26.53 %ID/g) over 177Lu-EB-PSMA-617 (29.89 ± 8.86 %ID/g) and 177Lu-PSMA-617 (4.28 ± 0.25 %ID/g) at 24 h post-injection. 225Ac-LNC1003 also showed very high tumor uptake (147.36 ± 27.56 %ID/g) in PC3-PIP tumor mice at 96 h post-injection. Radioligand therapy results revealed noteworthy tumor shrinkage after administration of a single dose of 18.5 MBq 177Lu-LNC1003 or 18.5 kBq 225Ac-LNC1003.
Conclusions: 177Lu/225Ac-LNC1003 were successfully synthesized and radiolabeled with high radiochemical purity and stability. With significantly improved absolute tumor uptake and retention, 177Lu/225Ac-LNC1003 have the potential to enhance therapy effect with lower dosages and less cycles, that promises clinical translation for the treatment of prostate cancer with various levels of PSMA expression.