Abstract
P317
Introduction: [18F]FE-PE2I is a promising radioligand for clinical PET imaging of the dopamine transporter (DAT) protein. The higher resolution and lower noise offered by PET imaging, compared to SPECT, may help detect disease induced alterations in small brain structures such as the substantia nigra (SN). In addition, due to the high extraction of [18F]FE-PE2I from blood, the early part of the scan can also provide information about brain perfusion. Here we report intermediate findings about DAT availability and perfusion alterations from an ongoing [18F]FE-PE2I PET study in patients with Parkinson’s disease (PD) and healthy controls (HC).
Methods: Twenty two PD subjects without dementia [12F/10M, mean age (sd): 63 (6) y, mean MDS-UPDRS Part III: 31 (12), mean disease duration: 5.8 (3.8) y, Hoehn and Yahr Scale: 2] and fourteen age and sex matched HC underwent [18F]FE-PE2I PET (mean injected activity: 149 (34) MBq) on the HRRT. All dopaminergic medications were withheld on the day of PET imaging. BPND and R1 were calculated from SRTM2 fits to the time activity curves (ref = cerebellum) for the SN, ventral striatum (VS), caudate and putamen. For R1, in addition to the aforementioned regions,the four cortical lobes were also included. For each brain region, Pearson correlation coefficients were calculated between motor severity scores (MDS-UPDRS-III) and the PET outcome measures. To investigate asymmetric nature of brain alterations due to PD, asymmetry indices (AI) for each PET outcome measure were computed for the PD cohort. AI was defined as (C - I) / 0.5 (C + I) , where C and I represent the PET outcomes on the contralateral and ipsilateral side of the brain, with respect to the symptom onset side. In addition to testing for regional asymmetry, associations between AI and motor severity scores were also investigated. All correlations were considered significant for p < 0.05, uncorrected.
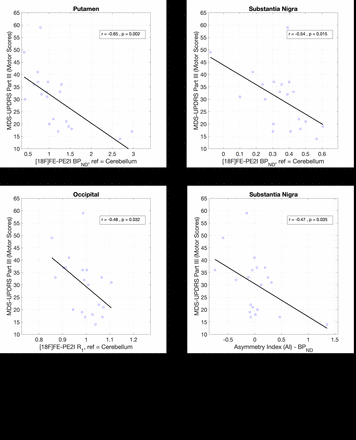
Results: Significantly lower BPND values were observed in PD, compared to HC, in the putamen (-65%), caudate (-56%), SN (-39%) and VS (-33%) (p<0.001, two-sample, two-tailed t-tests). Further, BPND in the posterior putamen was 33% lower compared to the anterior putamen after adjustment for the difference observed in HC. For R1, significantly lower values were observed in PD in the caudate (-14%, p=0.03) and occipital lobe (-5.0%, p=0.03), while the reduction in the parietal lobe was close to significance (-6.3%, p=0.06). Similar R1 findings have been observed with [11C]UCB-J R1 in a separate analysis that included all subjects reported in this study. In the PD cohort, significant negative correlations were observed between motor severity scores and [18F]FE-PE2I BPND in the putamen (r=-0.65, p=0.002) and SN (r=-0.54, p=0.01) but not the caudate (Fig 1 A,B). Significant negative correlations were also observed between motor scores and [18F]FE-PE2I R1 in the occipital lobe (r=-0.48, p=0.03, Fig 1C), while the correlation in the parietal lobe was close to significance (r=-0.44, p=0.05). Significant asymmetry in BPND was observed only in the putamen (AI=0.20 or 20% higher BPND in the contralateral putamen, p<0.001), with similar magnitude of asymmetry in the anterior (AI=0.19) and posterior (AI=0.22) parts. Though a significant asymmetry in BPND was not observed in the SN, AI in the SN (but not the putamen) was found to have a significant negative correlation with motor severity scores (Fig 1D). Neither any regional asymmetry, nor association of AI with clinical scores, was observed for regional R1 values.
Conclusions: [18F]FE-PE2I PET can simultaneously provide important information about DAT availability and brain perfusion in PD. Future work should investigate the prognostic value of [18F]FE-PE2I PET in longitudinal follow-up of PD subjects.