Abstract
P305
Introduction: The arterial blood samples collected during dynamic PET acquisition are vital for determining radiotracer blood clearance, and radiotracer metabolites, and constructing the metabolite-corrected arterial input function (AIF) for kinetic modeling. Arterial blood sampling is, however, a laborious and invasive procedure with possibilities of missed data points due to, for example, failure of the arterial line or sampling error. A method of generating the AIF accurately even with missing data and/or a reduced number of blood samples would be of benefit. One solution is to use population-based AIF scaled by a few measured data points, and yet the population-based AIF methods do not allow change of the input function shape and are, thus, less accurate. A mixed effect model that accounts for both population information and interindividual variability could potentially provide an accurate estimation of AIF even with limited data points. In this study, we developed an AIF parameter estimation method based on a nonlinear mixed-effects model.
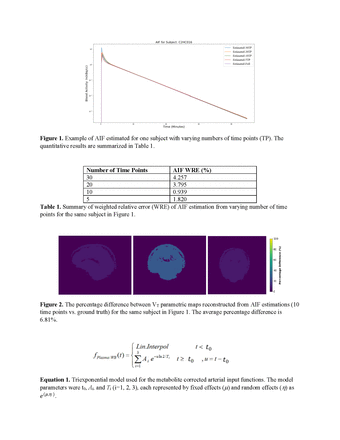
Methods: The study dataset (n = 52 healthy subjects) included 90-min dynamic brain PET data from studies that used the radiotracer 11C-DPA-713. The AIF data were acquired through arterial blood sampling at standardized time points post injection for radioactivity and radiometabolite measurements. A triexponential nonlinear mixed effect model was used to model AIF. The model parameters were expressed as a sum of fixed and random effects. Here, the fixed effects represent the population means and the random effects represent individual variation. The parameters were estimated using the stochastic approximation expectation maximization (SAEM) algorithm, which provided maximum likelihood estimates of the parameters. The method was validated using N+1 strategies, where N=51 were complete blood sample data with 40 time points and 1 was the test dataset not included in N. The test dataset contained reduced numbers of blood samples at randomly selected time points (5, 10, 20, or 30). The estimated results from the reduced-time point dataset were compared with ground truth using all time points. The weight relative error (WRE) between modeled AIF using reduced time points and the modeled AIF using complete time points was evaluated. The weighting factor represents the sensitivity of kinetic modeling to the AIF at different time points. Next, Logan graphical analysis with different AIFs was used to generate parametric maps of total distribution volume (VT) and these parametric images were compared.
Results: The AIFs generated using the proposed nonlinear mixed effect model and reduced (5-30) time points closely resembled the ground truth. The weighted relative errors remained below 10%. The reconstructed VT parametric map based on predicted and ground truth AIF showed minimal difference visually and quantitatively. In a representative test subject, the averaged voxel difference of all reduced time points between reconstructed from AIF estimations and ground truth is 18.57 ± 9.40%. Parameter estimation of AIF using the nonlinear mixed effect model is a promising method to overcome possible limited or lost data points in arterial blood sampling.
Conclusions: Parameter estimation of AIF using the nonlinear mixed effect model is a promising method to overcome possible limited or lost data points in arterial blood sampling.