Abstract
P232
Introduction: Stroke is the leading cause of lives lost with a significant impact on healthcare expenditures across the world as well, of which ischemic stroke dominates, about 70%, with a very high mortality rate and disability rate. Currently, oxidative stress is widely accepted as an important cause in the pathological process of ischemic/reperfusion (I/R) injury leading to cell damage and death via reactive oxygen species (ROS). Fe-GA CPNs (iron-gallic acid coordination polymer nanodots) indicated potential promise as an approach for stroke treatments in our previous work. Therefore, in this study, Fe-GA CPNs were further applied as ROS scavengers to treat I/R injury monitored by PET/MR and pathological examinations.
Methods: Fe-GA CPNs were successfully synthesized and characterized based on our previous method. The ·OH generated by the classic Fenton reaction was detected by electron spin resonance (ESR) using 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO), a radical trapping agent. In order to evaluate the cytotoxicity of Fe-GA CPNs and the protection from H2O2 stimulated cells in vitro, human umbilical vein endothelial cells (HUVEC) cells and mouse monocyte-macrophage leukemia cells (RAW264.7) were used as cellular modal, and the relative survival rate of cells was tested using methyl thiazolyl tetrazolium (MTT). Middle cerebral artery occlusion (MCAO) models which mimic clinical progress during a stroke were used for further in vivo evaluation. 2,3,5-triphenyl tetrazolium chloride (TTC) staining and H&E staining were utilized to evaluate the treatment effect of cerebral infarction. The activation levels of the Nrf2/HO-1 pathway and the Akt pathway were tested by western blot assay to better evaluate the underlying mechanism of Fe-GA CPNs. Furthermore, in the MCAO animal model, the expression of Nrf2 and HO-1 in brain tissue was evaluated qualitatively and quantitatively using immunofluorescence staining and the nuclear translocation of Nrf2 in the damaged brain tissue. In vivo biosafety of Fe-GA CPNs was also evaluated with H&E staining and hematological test.
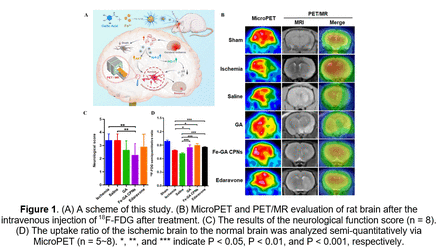
Results: The ESR spectrum revealed that the ultrasmall Fe-GA CPNs with ultrasmall size scavenged ROS efficiently. In vitro experiments showed that Fe-GA CPNs could protect cell viability after being treated with hydrogen peroxide (H2O2) and displayed the effective elimination of ROS by Fe-GA CPNs, which subsequently restores oxidation balance. In animal studies, neurological recovery was observed and the expected improvement of 18F-FDG uptake was effectively shown by microPET and PET/MR, which was proved by 2,3,5-triphenyl tetrazolium chloride staining. Furthermore, immunohistochemistry staining indicated that Fe-GA CPNs inhibited apoptosis through protein kinase B (Akt) restoration, whereas western blot and immunofluorescence indicated the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) pathway following Fe-GA CPNs application. Biosafety results demonstrated that Fe-GA CPNs hold good biocompatibility, and their toxicity to organisms is also negligible.
Conclusions: Fe-GA CPNs provide a satisfactory neuroprotective effect with improved cellular viability, reparation of neurological function and glucose metabolism, and apoptosis reduction being observed through cellular viability assays, functional scale, PET/MR imaging, and pathological examinations. They also effectively optimize the physical and chemical properties of GA while still exerting their corresponding chemical and biological activities. The excellent therapeutic potential of Fe-GA CPNs revealed its potential for clinical ischemia stroke treatment.