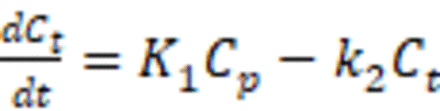Abstract
P1539
Introduction: Quantification without directly measuring an arterial input function (AIF) is vital for large PET neuroimaging studies. When a suitable reference region exists, kinetic parameters can be estimated with a reference region method such as the simplified reference tissue model (SRTM). The goal of this work is to investigate using SRTM estimates to derive an unscaled input function without blood sampling and examine possible scaling schemes for estimating volumes of distribution (VT). This could prove useful for VT quantification without arterial blood sampling.
Methods: Eight healthy participants (4 M, 4 F; Age=42 ± 27 years) were scanned for 90-120 min with the HRRT after injection of 513 ± 265 MBq [11C]LSN3172176, which is highly specific for muscarinic 1 acetylcholine receptors, and a metabolite corrected AIF was measured. SRTM was performed on a set of ROIs (Amygdala, Caudate, Pallidum, Parietal cortex, and Putamen) using either the Cerebellum as an ideal reference region or the Thalamus as an imperfect reference region. The shape of the input function was derived by solving the one tissue compartment model equation for CP with SRTM based parameter estimates. These input function shapes were estimated twice, first with ‘gold standard’ k2 from the 1TCM solution and R1 from SRTM, and a second time with both parameters estimated from SRTM for a more ‘real world’ evaluation. To survey potential scaling schemes for final estimation of VT, the derived input function curve was scaled using the ratio between the integral from zero to an early time point of the true AIF and the derived input function curve. Using this scaled input function, VT values were compared to VT values estimated from using the AIF with a 1 tissue compartment model.
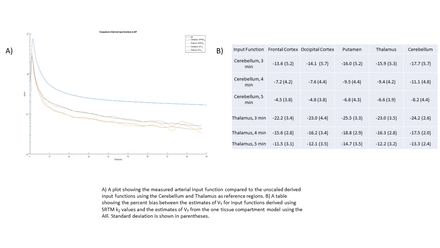
Results: SRTM estimates of k2,ref were 0.088 ± 0.021 min-1 when using the Cerebellum as a reference region and 0.055 ± 0.009 min-1 when using the Thalamus. This compares to the ‘gold standard’ values from the 1TCM of 0.097 ± 0.023 min-1 for the Cerebellum and 0.063 ± 0.013 min-1 for the Thalamus. The resulting input functions before scaling are plotted in part a of the figure along with the corresponding AIF. SRTM2 underestimated the k2 value of the Cerebellum by 9.3% ± 8.7% and the Thalamus by 11.9% ± 6.7%, which had a noticeable effect on the shape of the input function. Bias of estimates of VT values depended on the time point used in the scaling. When using the ‘gold standard’ k2 estimates, taking the ratios of integrals to 4 minutes provided the best tradeoff between an early time point and less biased mean estimates of VT over 5 ROIs (Cerebellum, Frontal Cortex, Occipital Cortex, Putamen, and Thalamus) with a bias of -2.0% ± 4.8% (mean and standard deviation across all participants and ROIs) with a scaling factor of 2.6 ± 0.7 when using the Cerebellum as a reference region and -9.2% ± 7.4% with a scaling factor of 2.3 ± 0.6 when using the Thalamus as a reference region. When using SRTM k2 estimates, ratios of the integrals to 5 minutes provided the best tradeoff between an early time point and bias in mean estimates of VT with a bias of -9.4% ± 5.6% and a scaling factor of 2.7 ± 0.7 when using the Cerebellum as a reference region and a bias of -12.1% ± 7.3% with a scaling factor of 2.4 ± 0.6 when using the Thalamus as a reference region.
Conclusions: The results of SRTM could be used to estimate the shape of the AIF. However, biased estimates of k2 alter the shape of the derived input function. Scaling 5 minutes of the integrated input function provided the best bias/variance tradeoff for VT estimation using both ideal and imperfect reference regions. Future work will examine image derived methods of scaling the input function and evaluate method performance for tracers that are modeled by a two tissue compartment model.