Abstract
P1305
Introduction: Carbon-11 labeled aminoisobutyric acid ([11C] AIB) is a tumor-seeking PET tracer which is a non-metabolized non-natural amino acid and is transported into cells predominantly via the system A amino acid transporter. [1-11C]alpha-AIB ([1-11C]AIB) was developed in the 1980s, and its usefulness was reported in the diagnosis of malignant melanoma. However, several problems have yet to be solved regarding its complicated procedure of radiosynthesis and cyanide residues in the final product. We have developed a novel radiosynthesis of 2-amino-[3-11C]isobutyric acid ([3-11C]AIB) and basic research of [3-11C]AIB PET as a preclinical imaging study. This study aims to investigate the biodistribution and radiation exposure of [3-11C]AIB in healthy adult males.
Methods: [3-11C]AIB was radiosynthesized using the previously reported method in our laboratory. For the PET imaging study, six healthy adult males (24-34 years old, median age 28) received a saline solution of [3-11C]AIB (mean dosages of 367 MBq) intravenously. PET/CT scans were performed using a GE Discovery MI 5-ring digital PET scanner. Immediately after injection of [3-11C]AIB, dynamic PET imaging of the heart and upper abdomen were performed for four minutes. From 5 minutes to 90 minutes, a total of 17 whole-body scans with a length of 4 minutes were obtained every 5 minutes. Brain, salivary glands, heart wall, heart contents, lungs, thyroid, liver, pancreas, spleen, adrenals, kidneys, prostate, and red bone marrow were manually marked with Volumes of Interest using each PET/CT data set. To determine the biodistribution of the radiotracer using PMOD 4.2, individual organ time-activity curves were first created. Then, IDAC-Dose 2.1 was used to evaluate the whole-body effective dose. Vital signs and laboratory exams were monitored before and after the radiotracer injection to assess the safety evaluation.
Results: All six subjects had no apparent symptoms or abnormalities in vital signs during or after the examination. In addition, the blood tests observed no renal or hepatic function abnormalities.
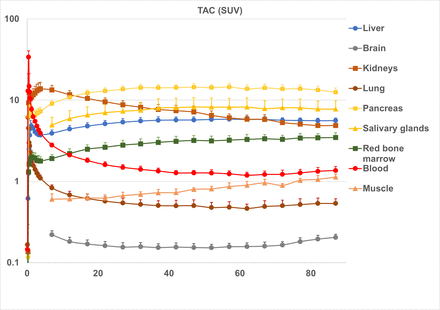
The accumulation of the radiotracer of the heart contents(blood) reached a SUVmean of 33.9±6.7 at 30 seconds after injection, then rapidly decreased to a SUVmean of 1.6±0.14 at 20 min.
There was also a substantial accumulation of the radiotracer in the kidneys, rising to SUVmean 14.8±2.8 at 4 min, followed by migration of the radiotracer from the pyelonephritic ureter to the urinary bladder. Accumulation in the kidneys at 20 min was SUVmean 9.6±1.1. Therefore, the kidneys were considered to be the principal eliminating organ of this radiotracer.
The accumulation in the pancreas and salivary glands at 20 min was SUVmean 13.0±1.2 and SUVmean 7.0±1.8, respectively. The substantial accumulation in the pancreas and salivary glands is similar to that of amino acid PET tracers such as [11C]methionine. The accumulation in the liver in the 20-min image was SUVmean 5.1±0.5. There was little the radiotracer migration to the bile ducts. The uptake in the brain and lungs was weak, with SUVmean 0.16±0.02 and 0.57±0.08 at 20 min, respectively.
The average effective whole-body dose based on the MIRD method was 5.08±0.32 μSV/MBq, estimated as 1.88±0.12 mSv from the administration of 370MBq of [3-11C]AIB.
Conclusions: [3-11C]AIB PET provided satisfactory image quality with adequate radiation absorbed dose and no side effects associated with its administration. Therefore, it has the potential to be useful in oncology imaging.