Abstract
P1222
Introduction: 177Lu-PSMA-617 is a radioligand with high affinity for prostate-specific membrane antigen (PSMA), enabling targeted β-irradiation of prostate cancer. We aimed to assess the outcome of patients with metastatic castration-resistant prostate cancer treated with 177Lu-PSMA-617 post-approval.
Methods: A retrospective review of patients who received 177Lu-PSMA-617 therapy at our institution was performed. Demographic characteristics, clinical and laboratory values, and post-treatment outcomes were reviewed and compared. RECIST 1.1 and CTCAE version 5.0 guidelines were used to evaluate radiographic response and to grade treatment toxicities.
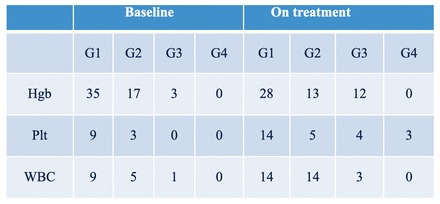
Results: Of the 61 patients treated, 25 patients had RECIST measurable disease, of which 8 had progressive disease, 11 stable disease, 5 partial response, and 1 complete response (ORR=24%). 31 (51%) patients developed a PSA50 response and 41 (67%) developed a PSA30 response (Figure 1). The average best PSA response was 45%. Broken down by site of metastases, the average PSA response in patients with bone metastases was 53% (n=21), with nodal metastases 29% (n=3), and with bone and nodal disease 39% (n=37). 17 patients reported grade 1 dry mouth and 8 reported grade 2 dry mouth. White blood count decreased by 33% and 31 patients developed leukopenia (14 grade 1, 14 grade 2, 3 grade 3). Platelet count decreased by 30% and 26 patients developed thrombocytopenia (14 grade 1, 5 grade 2, 4 grade 3, 3 grade 4). Hemoglobin count decreased by 9% and 53 patients developed anemia (28 grade 1, 13 grade 2, 12 grade 3) after 177Lu-PSMA-617 therapy (Table 1).
Conclusions: Initial experience with PSMA Radioligand Therapy mirrors the results seen in the VISION trial with PSA50 response seen in 51% of patients and an ORR of 24%. The most common side effects seen were anemia and leukopenia.