Abstract
P1213
Introduction: Pancreatic ductal adenocarcinoma (PDA) is a deadly disease and its characteristic desmoplastic stroma, constituting 60–70% of its volume, is one of the factors that contributes to the dismal outcomes. Fibroblast activation protein (FAP)-expressing Cancer-Associated Fibroblasts (CAFs) are one of the most important constituents of the tumor microenvironment (TME) because they play a fundamental role in the carcinogenesis, fibrosis, tumor growth, metastases, and treatment resistance. Lack of noninvasive tools for in vivo temporal and spatial profiling of CAF identity and function is a critical barrier for translation of existing knowledge of TME to address the unmet clinical needs. [68Ga]FAP-inhibitor (FAPI)-46 has emerged as a PET radiotracer with optimal properties for FAP-targeted clinical imaging and theranostics in PDA. We present the design and available results from an ongoing Phase 2, multicenter, single arm, open label, non-randomized study (registration#NCT05262855) of [68Ga]FAPI-46 PET in resectable or borderline resectable PDA.
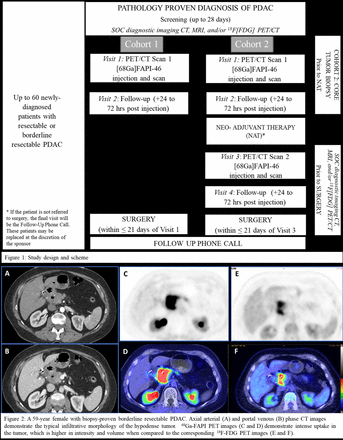
Methods: This study will enroll up to 60 adult treatment-naïve patients with pathologically confirmed PDA who are candidates for curative-intent surgical resection without (Cohort 1) or following neoadjuvant treatment (NAT) (Cohort 2) (Figure 1). Baseline PET will be acquired from vertex through mid-thighs at 15-minutes (±10) after intravenous administration of 5 mCi (± 2) [68Ga]FAPI-46. Patient fasting or blood glucose measurements are not needed for [68Ga]FAPI-46 PET. An additional scan will be obtained in Cohort 2 patients at the completion of NAT but prior to surgery. Truth standard is histopathology and FAP immunohistochemistry (IHC) of the resected tumor specimens. Primary study objective is the sensitivity, specificity and accuracy of [68Ga]FAPI-46 PET for FAP-expression with the secondary objective being its predictive values, and further validation of its safety profile. Exploratory objectives include detection of metastases against a composite of biochemical, standard-of-care imaging, and histopathology reference, and comparison of pre- vs. post-NAT PET metrics in Cohort 2. Two sites are currently open to recruiting subjects. Three more sites are in the activation process.
Results: Eight eligible patients have been consented. Three consented patients could not undergo [68Ga]FAPI-46 PET due to logistical reasons (n=2) or the need for surgery prior to availability of [68Ga]FAPI-46 PET. Five consented subjects (mean age: 69.2years; range:59-79; 3 females), all from Cohort 2, have undergone the baseline pre-NAT [68Ga]FAPI-46 PET scan. None of the patients experienced adverse side-effects to [68Ga]FAPI-46 injection. All tumors were in pancreatic head-neck (mean length: 3.4cm; range: 1.5-4.1cm) and showed intense [68Ga]FAPI-46 uptake (mean SUVmax 17.2; range: 15.9-19.5; mean SUVmean 9.8; range: 8.9-11; mean tumor-to-liver SUVmax ratio: 6.6; range: 3.3-10.2). Although the uninvolved pancreas also showed diffuse and moderate [68Ga]FAPI-46 uptake, the tumor-to-pancreas SUVmax ratio was high (mean=3; range: 1.0-7.7). [68Ga]FAPI-46 uptake in the tumors was higher compared to standard-of-care [18F]FDG (n=3) (mean FDG SUVmax 8.9, range: 5.7-10.9; mean FDG SUVmean 5.9, range: 3.8-7; mean tumor-to-liver FDG SUVmax ratio: 2.7, range: 1.7-3.5) but lower when compared to the uninvolved pancreas on the respective scans (mean tumor-to-pancreas FDG SUVmax ratio: 5, range: 3.8-5.7). None of the patients had confirmed metastases on initial staging scans.
Conclusions: For the potential clinical translation of a theranostic radiotracer such as [68Ga]FAPI-46, the ongoing trial seeks to evaluate a coherent development strategy to reduce the risk and raise the likelihood of meeting FDA standards and consumer expectations. Although it is early to draw definite conclusions, given their mechanistic and operational differences, [68Ga]FAPI-46 has the potential to be complementary to [18F]FDG in patients with PDA.