Abstract
P1158
Introduction: One of the challenges to the successful design and implementation of HIV curative strategies is the limited ability to accurately quantify and characterize the whole-body burden of HIV-1 infection. In addition, there is an urgent need to identify anatomical areas of HIV-1 infection responsible for viral rebound after antiretroviral therapy (ART) is stopped, as a majority of people with HIV (PWH) experience HIV rebound in blood within weeks of stopping therapy. We developed a zirconium-89 labeled broadly neutralizing antibody (bnAb), 89Zr-VRC01, that binds to the HIV-1 envelope gp120 protein and is able to identify viral protein production in viremic PWH and persistent infection in PWH on suppressive ART. Here we evaluate 89Zr-VRC01 to measure tissue HIV-1 activity during analytical treatment interruption (ATI) prior to and just following detectable viremia with the goal of identifying areas of emerging virus responsible for subsequent systemic dissemination.
Methods: 89Zr-VRC01 was produced at the UCSF Radiopharmaceutical Facility and was released for patient administration following quality control (QC) testing. 5 PWH on long-term ART participating in a treatment interruption protocol were enrolled in our investigational review board/FDA approved clinical study. 89Zr-VRC01 (~1 mCi) was given a median of 21 days following cessation of ART. PET/MR (SIGNA, GE Healthcare) imaging was performed 3 days following tracer injection. Ratios of 89Zr-VRC01 standardized uptake values between the blood pool and tissue regions of interest (ROI) were calculated using Osirix MD (Pixeo) to measure mean and maximum SUV values from 3-dimensional and isometric gates.
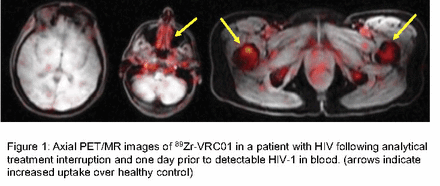
Results: 89Zr-VRC01 injections were well tolerated without any major adverse events or infusion reactions. Overall, tracer uptake was higher in various lymphoid tissues (nasal-associated lymphoid tissue [NALT], inguinal and axillary LN, tonsillar tissue), bowel wall, axial/peripheral bone marrow and spleen in all participants compared to HIV-uninfected control participants. We were able to perform PET/MR imaging in a female participant 1 day prior to first detectable blood viral load measurement (3 days following 89Zr-VRC01 injection). Unlike viremic PWH in which 89Zr-VRC01 uptake is seen increased across many lymphoid and other tissues, we observed much higher but very focal levels of 89Zr-VRC01 in ROIs including specific inguinal lymph nodes (rSUV 0.5; HIV bNAbs have much higher blood distribution compared to tissues in people without HIV), bowel wall (rSUV 1.6), bone marrow (rSUV1.6) and spleen (rSUV 1.74). In prior studies of viremic, ART suppressed and uninfected controls, we observed no 89Zr-VRC01 uptake in the brain/central nervous system (CNS; rSUV all <0.2). However, we identified diffuse but patchy signal in brain (rSUV 1.7) just prior to detectable HIV in blood. Figure 1 shows examples of axial PET-MRI 89Zr-VRC01 uptake in an ATI participant imaged just prior to detectable HIV-1 in blood and an uninfected control participant.
Conclusions: This is the first immunoPET imaging study to characterize areas of active HIV infection following ART cessation but prior to viral rebound in blood. 89Zr-VRC01 PET-MR imaging was sensitive to detect early foci of HIV activity in tissue prior to detectable plasma HIV-1 RNA and wide-spread dissemination. Our data suggest that tissue HIV reservoirs that persist on long-term ART and are responsible for viral recrudescence may be focal or arise from anatomical locations not previously appreciated (e.g. bone marrow, brain). Finally, uptake in brain prior to detectable viremia suggests either trafficking of infected cells to the CNS or early reactivation of HIV-infected CNS-resident cells.