Abstract
P1147
Introduction: Immune modulating agents are becoming the mainstay for treatment of a variety of malignancies, but only a subset of patients respond to immunotherapy. LAG-3, a cell surface marker predominantly expressed on CD3-positive T cells, is an immune inhibitory receptor which negatively regulates T cell proliferation, activation, and proinflammatory cytokine production. LAG-3 plays a role in suppressing the activity of a subset of PD-1 expressing T cells; a large fraction of PD-1 positive CD8+ and CD4+ tumor infiltrating lymphocytes are noted to co-express LAG-3. Diffuse large B-cell lymphoma (DLBCL) expresses very high levels of LAG-3. In lymphoma patients, PD-1 or PD-L1 inhibitors have only modest activity as monotherapy, suggesting combination approaches targeting additional immune inhibitory receptors such as LAG-3 may improve tumor response to therapy. Non-invasive Positron Emission Tomography (PET) imaging of LAG-3 expression can provide new insights in the mechanisms of immunotherapy with LAG-3 inhibitors and potentially predict treatment responses. In this pilot study, we evaluated safety and feasibility of PET/CT imaging with a radiolabeled monoclonal antibody (mAb) against LAG-3 (89Zr-DFO-REGN3767) (NCT04566978) in patients with anti-PD-1/PD-L1 naïve relapsed/refractory DLBCL with either progression or who were not candidates for autologous stem cell transplant and were to undergo treatment with anti-LAG-3 mAb (REGN3767) and cemiplimab (NCT03005782).
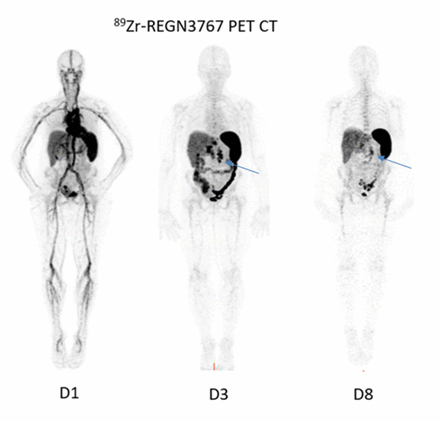
Methods: The prospective imaging study (LAG-3 iPET) included cold antibody mass escalation (2 mg, 5 mg and 10 mg) co-infused with 1 mCi of 89Zr-DFO-REGN3767 followed by PET/CT scans at day 1, 2-4 and 5-7 post injection (PI). Multiple Serum samples were obtained on the day of and at each imaging time point. All patients were monitored for vitals and side effects during and after the infusion up to the last time point of imaging. Biodistribution and uptake in normal organs, and tumor lesions was evaluated. The optimal single time point for imaging was evaluated by examining uptake in target-expressing vs non-target expressing tissues.
Results: 89Zr-DFO-REGN3767 infusion in 7 patients (3 pts each at 2 and 5mg; 1 patient at 10 mg), was well tolerated and no immediate or delayed adverse effects were seen in any patient after injection. Activity in the serum cleared biexponentially with T1/2b (α) 3.5 h ±2.1 and T1/2b (β) 37 h ±11.3. Whole body clearance was also mono exponential with T1/2b of 63.0 ±7 h.
Prominent uptake was seen in spleen, followed by liver and kidneys with mean SUV of 30.8, 3.5 and 2.1 at 133 h PI, respectively. Marrow uptake was low (1.7 % ID/kg). Normal lymph node uptake was not prominently noted. Uptake in tumor lesions was seen in 6/7 patients including nodal or bone lesions. Most optimal visualization of lesions based on Tumor/Background ratio and SUV was at D5-8 for most lesions. Maximum lesion uptake, evaluated in 6 target lesions ranged between 8.9 to 24.3.
Conclusions: 89Zr-DFO-REGN3767 imaging is feasible, has favorable biodistribution and allows for non-invasive PET targeted imaging and detection of LAG-3 expressing tumor.