Abstract
P1126
Introduction: Stereotactic body radiation therapy (SBRT) and 90Y selective internal radiation therapy (SIRT), are established treatments for unresectable hepatocellular carcinoma (HCC) and secondary hepatic metastases. While both modalities have been shown to be safe and effective, they also have well-demonstrated clinical challenges and limitations. For SBRT, avoidance of normal liver parenchyma can be challenging in the presence of large or multifocal disease. Therefore, when tumor burden is high and safe SBRT delivery is limited by the acceptable normal tissue doses, SIRT remains the preferred treatment approach. Although SIRT preferentially spares healthy liver parenchyma through arterial blood supply selection, SIRT can also result in heterogeneous dose coverage due to hemodynamics and irregular tumor perfusion. To overcome these treatment limitations, this Phase I clinical trial aims to evaluate the safety of using 90Y PET/CT-based absorbed dose maps to guide a dose-painting approach in SBRT in which tumors or tumor subregions that are underdosed by initial 90Y SIRT are sequentially treated with SBRT.
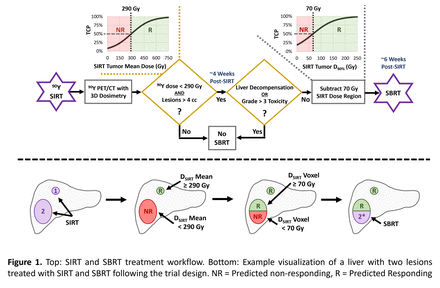
Methods: From September 2020 through December 2022, 19 patients completed treatment and post-SIRT dosimetry after informed consent as part of an IRB-approved Phase I clinical trial (NCT04518748). As shown in Figure 1, all patients received standard-of-care 90Y SIRT for the treatment of unresectable HCC lesions or secondary metastases. Post-SIRT dosimetry was performed using 90Y PET/CT to generate 3D absorbed dose maps and calculate mean absorbed doses to individual lesions, segmented on baseline anatomical imaging by a radiologist. Based on prior tumor control probability (TCP) modeling, lesions were considered eligible for SBRT treatment if the mean absorbed dose with recovery coefficient applied was less than 290 Gy (50% TCP). Following co-registration of 90Y dose maps with the SBRT planning CT, SBRT tumor volumes were cropped at the voxel level to eligible-lesion subregions that received less than 70 Gy from SIRT (50% TCP based on separate dose coverage modeling). If further clinical criteria was met, SBRT-eligible targets were treated approximately 6 weeks post-SIRT with conventionally planned photon SBRT. Safety of the treatment approach was assessed by evaluating changes in measures of liver function, performance status, and tracking adverse events.
Results: 90Y PET-based mean lesion absorbed doses from SIRT was on average 508 Gy (range -4479) for 48 lesions segmented on 19 patients. Of these 19 patients, 10 had lesions (28 lesions total) that were considered dosimetrically eligible for SBRT based on the above criteria of 290 Gy mean absorbed dose to lesions from 90Y. Of those, 8 patients (12 lesions total) received SBRT with external beam doses ranging from 35-50 Gy delivered in 5 fractions; one patient was excluded from SBRT based on positive tumor response to SIRT, and another was excluded based on disease progression prior to SBRT. As of December 2022, 6-month follow up data was available for 6/8 patients who received SBRT. At 6 months, two patients had an increase in Child-Pugh score of at least 2 points and two patients had a change in ALBI >= 0.5. ECOG performance status grade increased from 0 to 1 for two patients and 0 to 2 for another patient. Two CTCAE grade 3 adverse events were reported following SBRT with one categorized as definitely related and one as probably related to treatment.
Conclusions: Interim, preliminary analysis of the trial suggests that sequential SIRT and SBRT using 90Y PET-based dose maps for SBRT targeting is a feasible and well-tolerated treatment approach for unresectable hepatic malignancies.
Acknowledgements/Disclosure: Research reported in this publication was supported by the National Cancer Institutes of Health under Award Numbers R01EB022075 and P30CA046592. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.