Abstract
P1013
Introduction: Therapeutics for neurodegenerative disease face challenges for brain delivery that include off-target effects, first-pass metabolism, and limited entry by the blood-brain barrier. The nose-to-brain (N2B) pathway is a direct transport pathway through the skull that avoids all the above and has generated interest as a delivery approach for promising therapeutics like N2B insulin for Alzheimer’s Disease. However, clinical implementation has been limited by a lack of methods to noninvasively determine the fate of N2B drugs following delivery and to troubleshoot or optimize dosing methods. We evaluated PET in non-human primates as a method to track N2B 18F-fluorobenzoyl-insulin delivery, nasal distribution, and uptake into brain.
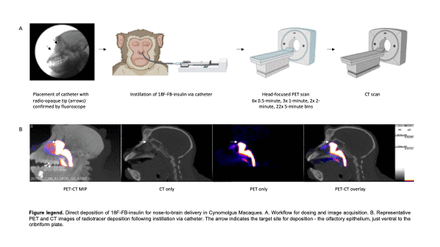
Methods: 18F-FB-insulin was prepared by reacting A1,B29-di(tert-butyloxycarbonyl)insulin with 18F-N-succinimidyl-4-fluorobenzoate. A rhinoscope was used to guide a catheter with a radio-opaque tip towards the cribriform plate for direct deposition of 18F-FB-insulin. Two methods of radiotracer delivery to the cribriform plate were evaluated: (1) In Rhesus Macaques (1M, 1F), 450 µL aerosolized 18F-FB-insulin was delivered via catheter-fitted nebulizer assembly with 0.5 mL/minute airflow (n=3 total); (2) 18F-FB-insulin was delivered as 613 µL solution to Cynomolgus Macaques (1M, 1F) via catheter-fitted syringe pump with 150 µL/minute flow rate (n=3 total). The change to Cynomolgus was required due to pandemic supply chain issues. Subjects were transferred to a head-focused PET scanner (Focus 220) after dosing to quantify 18F-FB-insulin deposition and transport over 120 minutes (6x 0.5 minute, 3x 1 minute, 2x 2 minute, 22x 5 minute PET bins); CT and MRI scans were collected separately. The PET images were reconstructed into static and dynamic series corrected for decay, scatter, and attenuation by CT. PET and CT images were co-registered, resampled to uniform voxel size (0.6 mm3), and used to quantify the fraction of radiotracer in the nasal cavity, cribriform plate, and brain using static scans in VivoQuant (inviCRO). Quantification of all dynamic scans and brain atlas regions are in progress.
Results: The average radiochemical yield of 18F-FB-insulin was 10.7% (± 2.9 SEM) determined by radio-HPLC of the isolated product. Liquid instillation delivered 366 µCi (± 242 SEM) to the subject, where 39.89% (± 7.175 SEM) of the dose was retained in the nasal cavity 8 minutes after dosing (scan start time). Radiotracer quantification for the whole brain and cribriform plate accounted for 0.949% (± 0.451 SEM) and 0.002% (± 0.0005 SEM) of the activity in the FOV, equivalent to 0.337% (± 0.128 SEM) and 0.0009% (± 0.0004 SEM) of the total delivered dose. On average, 18F-FB-insulin concentration in the brain was 17-fold higher than the background of the scan. Trends show uptake of 18F-FB-insulin in the Cynomolgus caudate nucleus, limbic lobe, and subthalamus. Quantification of brain atlas regions are in process. Only 0.1693% (± 0.0913 SEM) of the 18F-FB-insulin starting dose was quantified in the FOV for aerosol delivery subjects.
Conclusions: We successfully delivered 18F-FB-insulin to the brain of Cynomolgus Macaques using direct liquid instillation with a catheter placed close to the cribriform plate. Aerosol delivery was achieved but inefficient relative to liquid instillation. The catheter-based delivery approach combined with PET successfully tracked the fate of N2B-delivered 18F-FB-insulin and is thought to be broadly applicable for assessments of other therapeutic agents. With this technique, prevailing dose formulations, absorption enhancers, and/or delivery approaches can be identified and optimized to reduce the barriers to efficacious N2B therapeutics in the clinic.
Acknowledgements: This work is supported by NIH R21 1R21AG054960-01.