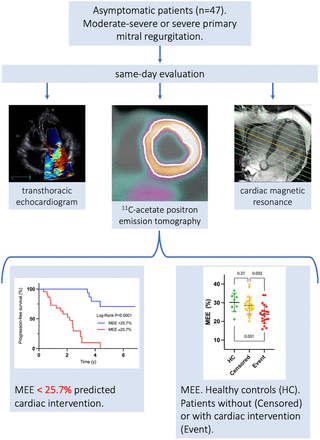
Visual Abstract
Abstract
Subjects with asymptomatic moderate-to-severe or severe primary mitral regurgitation are closely observed for signs of progression or symptoms requiring surgical intervention. The role of myocardial metabolic function in progression of mitral regurgitation is poorly understood. We used 11C-acetate PET to noninvasively measure myocardial mechanical external efficiency (MEE), which is the energetic ratio of external cardiac work and left ventricular (LV) oxygen consumption. Methods: Forty-seven patients in surveillance with mitral regurgitation and no or minimal symptoms prospectively underwent PET, echocardiography, and cardiac MRI on the same day. PET was used to simultaneously measure cardiac output, LV mass, and oxygen consumption to establish MEE. PET findings were compared between patients and healthy volunteers (n = 9). MEE and standard imaging indicators of regurgitation severity, LV volumes, and function were studied as predictors of time to surgical intervention. Patients were followed a median of 3.0 y (interquartile range, 2.0–3.8 y), and the endpoint was reached in 22 subjects (47%). Results: MEE in patients reaching the endpoint (23.8% ± 5.0%) was lower than in censored patients (28.5% ± 4.5%, P = 0.002) or healthy volunteers (30.1% ± 4.9%, P = 0.001). MEE with a cutoff lower than 25.7% was significantly associated with the outcome (hazard ratio, 7.5; 95% CI, 2.7–20.6; P < 0.0001) and retained independent significance when compared with standard imaging parameters. Conclusion: MEE independently predicted time to progression requiring valve surgery in patients with asymptomatic moderate-to-severe or severe primary mitral regurgitation. The study suggests that inefficient myocardial oxidative metabolism precedes clinically observed progression in mitral regurgitation.
Primary mitral valve regurgitation affects close to 2% of the overall population, increasing to 10% among the elderly. Surgical repair or replacement are the treatments of choice (1). Assessment of mitral regurgitation is an important task of cardiac imaging. In clinical routine, this is usually accomplished by integrating echocardiographic findings with the clinical picture (2,3). To determine the impact of moderate or severe mitral regurgitation on left ventricular (LV) structure and function, the current recommendation is evaluation of LV diameters or volumes and LV ejection fraction (LVEF), with cardiovascular MRI (CMR) being considered the gold standard for such evaluations (4). A novel and theoretically attractive measure of LV performance in valvular heart disease is myocardial mechanical external efficiency (MEE), based on PET. MEE relates the mechanical energetic output of the left ventricle, measured as forward cardiac output times mean arterial blood pressure, to its chemical input from oxidative acetate metabolism, measured by PET (5). MEE decreases in heart failure (6) and in symptomatic primary and secondary mitral regurgitation (7–9), but data are scarce.
Given the increasing prevalence of moderate and severe mitral regurgitation, and the increasing complexity of therapeutic options (valve replacement and surgical and different types of interventional repair, including percutaneous edge-to-edge repair), the need to detect an adverse impact on LV performance early has gained urgency. We therefore set out to evaluate the impact of severe asymptomatic primary mitral regurgitation on MEE, its relation to quantitative measures of mitral regurgitation magnitude and LV remodeling by echocardiography and CMR (the standard imaging techniques in mitral regurgitation), and the role of myocardial metabolic integrity in predicting time to progression mandating surgical intervention.
MATERIALS AND METHODS
In total, 47 asymptomatic or mildly symptomatic patients (class I or II according to New York Heart Association Functional Classification) confirmed by bicycle exercise testing to have severe degenerative and chronic primary mitral regurgitation by echocardiographic criteria (2) were evaluated and included in the study between October 2013 and March 2018 at the Department of Cardiology, Uppsala University Hospital. Patients with other concomitant moderate or severe valve disease, nonsinus rhythm, a history of coronary artery disease, chronic renal disease, symptomatic or severe lung disease, and method-specific contraindications were excluded. All patients underwent 11C-acetate PET, echocardiography, and CMR on the same day. CMR and PET were performed 1 h apart with no intake of food or fluids between scans to avoid hemodynamic alterations. Additionally, a group of healthy volunteers (n = 9) underwent same-day 11C-acetate PET and echocardiography. The healthy volunteers had no signs or symptoms of cardiovascular disease or other chronic diseases. The study was approved by the Regional Ethical Review Board at Uppsala University (diarienummer 2012/543), and all subjects provided written informed consent.
PET
PET/CT scanning was performed with a GE Healthcare Discovery ST16 or DMI20. After a scout CT scan, a low-dose CT scan (120 kV, 20 mAs) was performed. After this, a 27-min list-mode emission scan was performed, starting simultaneously with automated injection of a 5-MBq dose of 11C-acetate per kilogram of body weight as a 5-mL bolus (1 mL s−1) in an antecubital vein, followed by a 30-mL saline flush (2.0 mL s−1). The collected list-mode emission data were used to create a dynamic image series consisting of 29 time frames using all data with 5-s frame lengths during the first minute. PET data were analyzed using software developed in-house (8) with full automation (Fig. 1).
Automatic postprocessing of cardiac 11C-acetate PET/CT images. (A) First-pass analysis after intravenous bolus injection of a few micrograms of 11C-acetate. Arterial clusters (red) indicate left atrium, left ventricle, and aorta, whereas venous clusters (blue) indicate vena cava, right atrium, right ventricle, and pulmonary artery. (B) Time–activity curves of clusters for arterial and venous blood, and corresponding isolated first-pass peaks, from which cardiac output and external work (cardiac output × mean arterial pressure) are calculated as in reference 11. (C) LV mass is measured by delineating LV endo- and epicardial contours by thresholding myocardium on parametric image representing myocardial blood flow (12). (D) Kinetics of radioactive content over time in myocardium is measured from region in C (8). 11C-acetate is trapped intracellularly as 11C-acetyl-coenzyme A and converted to 11C-CO2 by myocardial mitochondriae; washout rate of radioactivity is directly proportional to mean myocardial oxygen uptake. Total LV oxygen consumption is measured as mean myocardial oxygen uptake × LV mass. External work and total LV mean myocardial oxygen uptake are converted to Joules, the ratio of both yields MEE (8).
MEE was calculated by a standard formula (incorporating caloric conversion factors) as proposed by Bing et al. in 1949 (10): where MAP is mean arterial pressure (mm Hg), SV is stroke volume (mL/beat), HR is heart rate (beats/min), MVO2 is mean myocardial oxygen uptake (mL/g/min), and LVM is LV mass (g).
where MAP is mean arterial pressure (mm Hg), SV is stroke volume (mL/beat), HR is heart rate (beats/min), MVO2 is mean myocardial oxygen uptake (mL/g/min), and LVM is LV mass (g).
The dynamic PET dataset was used to measure forward cardiac output (aortic flow) with an indicator dilution approach, as previously described (8,11). Heart rate and blood pressure were measured noninvasively at the time of PET scanning. Heart rate was used to calculate forward stroke volume from forward cardiac output. The full dynamic dataset was used to obtain the denominator of the MEE equation, mean myocardial oxygen uptake, and LV mass, as previously described (8,12). PET postprocessing was fully automated and produced identical results when iterated. Test–retest results using this technique were previously published, showing a 9% coefficient of variance for MEE in healthy volunteers (13).
Echocardiography
Echocardiography (Vivid-7; GE Vingmed Ultrasound AS) was performed according to current guidelines. All studies were performed by experienced sonographers and interpreted by a single experienced physician.
LV end-diastolic volume, LV end-systolic volume, and LVEF were assessed using the biplane Simpson method. Left atrial volume was calculated by the biplane area length method. LV end-diastolic volume, LV end-systolic volume, and left atrial volume were indexed to the body surface area. Total stroke volume was calculated as the difference between LV end-diastolic and end-systolic volumes. Aortic forward stroke volume was calculated using the Doppler velocity time integral method, using the aortic annulus diameter for LV outflow tract diameter. Mitral regurgitant volume was estimated by both the proximal isovelocity surface area method and the volumetric method (total stroke volume – forward stroke volume). LV global longitudinal strain was measured by strain rate imaging.
Cardiovascular Mitral Regurgitation (CMR)
CMR studies were performed using an Ingenia 3-T whole-body scanner (Philips Healthcare) with an 80 mT·m−1 gradient system. Short- and long-axis cine images were acquired using a steady-state free-precession pulse sequence. LV volumes and mass were manually segmented from short-axis stack images using long-axis images to define the basic slice. End-diastolic endocardial and epicardial contours were propagated, with manual readjustments performed as required. Papillary muscles and adnexal muscle tissue were included in LV mass. Phase-contrast images were acquired perpendicular to the proximal ascending aorta to quantify aortic flow (forward stroke volume), using a semiautomated algorithm. Images were analyzed using commercial software (CVI42; Circle Cardiovascular Imaging). Mitral regurgitant volume was calculated by subtracting aortic forward stroke volume from total LV stroke volume. End-systolic wall stress was estimated using the thick-wall sphere model (14), for which end-systolic cavity pressure was substituted with systolic brachial pressure obtained at PET. A CMR-based MEE (MEECMR/PET) was constructed using aortic forward stroke volume and LV mass from CMR with mean arterial pressure, HR, and mean myocardial oxygen uptake from PET.
Outcomes
For outcome analysis, patients were followed regularly at our clinic or affiliated hospitals until March 2021. Time from inclusion to mitral valve intervention was recorded. The decision for mitral valve intervention was at the discretion of a multidisciplinary conference and in most cases (19 of 22) triggered by a combination of echocardiographic progression of mitral regurgitation and heart failure symptoms.
Statistical Methods
Categoric variables are presented as number and frequency. Continuous variables are presented as mean ± SD or as median and interquartile range. Correlations were assessed using linear regression. The agreement of corresponding parameters from PET and CMR was studied using Bland–Altman plot analyses, and the significance of bias was studied with paired t tests.
PET results were compared between patients and healthy volunteers using t tests. The relation of outcome data toward MEE and standard imaging parameters was analyzed by univariate Cox proportional hazards. Multivariate Cox models were experimentally performed using the best MEE cutoff and significant univariate predictors from echocardiography (end-systolic volume, mitral regurgitant volume, maximum tricuspid jet velocity) or CMR (end-diastolic volume, end-systolic volume, mitral regurgitant volume).
P values of less than 0.05 were considered significant. Statistical analyses were performed using JMP, version 16 (SAS Institute Inc.), and Prism, version 9 (GraphPad Software).
RESULTS
Clinical and laboratory findings are shown in Table 1. The mean age of the study population was 59.4 ± 11.0 y, and 91% (n = 44) were men. All patients met echocardiographic criteria for severe degenerative mitral regurgitation at the time of inclusion. The most common valve defect was an isolated or dominant P2-segment prolapse of the posterior leaflet (78%, n = 39), followed by Barlow disease (14%, n = 7)
Baseline Patient Characteristics (n = 47)
A history of hypertension was present in 60% (n = 28). Symptoms were categorized as New York Heart Association class I in 89% (n = 42) and class II in 11% (n = 5) at referral. One patient did not complete the CMR scan because of claustrophobia; this patient did undergo PET. Healthy volunteers (n = 9, 54 ± 8 y, 3 men) had no history of cardiac disease and had normal echocardiographic findings.
Myocardial Efficiency
Table 2 shows the results of MEE and associated PET measurements in healthy volunteers, compared with mitral regurgitation patients. There were significant differences in age and sex distribution between the groups. Average MEE in patients (26.3% ± 5.3%) was significantly reduced compared with healthy volunteers (30.1% ± 5.0%, P = 0.048; Table 2). MEE in patients who reached an endpoint was lower than in censored patients (mean difference, −4.6%; 95% CI, −7.4 to −1.8; P = 0.002), whereas MEE in censored patients was similar to that in healthy volunteers (mean difference, −1.7%; 95% CI, −5.4% to 2.1; P = 0.37), as shown in Figure 2.
11C-Acetate PET/CT Comparison of Healthy Controls and Patients with Asymptomatic Severe Primary Mitral Regurgitation
(A) Plots of MEE comparing healthy controls (HC) and study patients who were followed without (censored) or with (event) progression mandating surgical intervention. (B) Kaplan–Meier plot showing that patients with MEE below 25.7% at inclusion required valvular surgery significantly sooner than patients with MEE above 25.7%.
The results of linear correlation analyses of MEE with parameters from echocardiography and CMR are given in Table 3, showing inverse weak but significant correlations with indices associated with regurgitation, remodeling severity, and LV global longitudinal strain. Notably, MEE did not correlate with LVEF or end-systolic wall stress.
Echocardiography and CMR: Mean Values, Linear Correlations with MEE, and Univariate Cox Proportional-Hazards Analysis
The MEE equation includes forward stroke volume and LV mass, here obtained by PET by an automated image analysis procedure. Both these parameters were also available from the same-day CMR in mitral regurgitation patients, and cross-modality correlations and agreement were good (forward stroke volume: r = 0.88 [95% CI, 0.79–0.93; P < 0.0001] and bias = −1 ± 8 mL [P = 0.52]; LV mass: r = 0.91 [95% CI, 0.84–0.95; P < 0.0001] and bias = 0 ± 15 g [P = 0.9]). When CMR-based forward stroke volume and LV mass were inserted into the MEE equation, the correlation remained good (r = 0.76; 95% CI, 0.61–0.86; P < 0.0001), but MEE values from PET alone were higher (bias = 4.6% ± 3.5%, P < 0.0001). A residual analysis showed that differences in both forward stroke volume and LV mass contributed significantly to MEE variance. Use of 2 different PET scanners in the study did not impact bias toward CMR-based MEE.
Clinical Outcomes
The median duration of follow-up was 2.7 y (interquartile range, 1.9–3.2 y). The endpoint of surgical intervention was reached in 21 subjects. One subject experienced disease progression and developed characteristic symptoms. Intervention was recommended, but the patient rejected surgery; cardiovascular death occurred 5 y after inclusion, and the time point of recommendation for surgery was used as an endpoint surrogate. Thus, the final number of subjects considered reaching the endpoint in statistical analyses was 22.
The indication for intervention was at the discretion of the treating physicians and followed guidelines available at the time of the study (15). Occurrence of symptoms during surveillance was noted in 17 of 22 (77%) in whom surgery was eventually recommended.
Univariate analysis showed that MEE as a continuous variable predicted outcome; a decrease in MEE by 1% increased the relative hazard of the outcome within the next year by 19% (hazard ratio, 0.84; 95% CI, 0.75–0.93; P = 0.0004). ROC analysis provided an MEE cutoff for event prediction at 25.7%, close to the lower limit of normalcy, which was associated with a risk ratio of 7.5 (95% CI, 2.7–21; P < 0.0001) in a univariate Cox model. A Kaplan–Meyer plot is shown in Figure 2B.
Table 3 shows univariate baseline predictors of outcome by Cox proportional-hazards analysis from echocardiography and CMR. MEECMR/PET performed similarly to MEE from PET alone, both as a continuous variable (hazard ratio, 0.85; 95% CI, 0.77–0.93) and as a binary cutoff, established as an MEECMR/PET of less than 22.2% (hazard ratio, 5.9; 95% CI, 2.0–17.8).
The echocardiographic and CMR-based severity of mitral regurgitation were also predictive (P < 0.05) in univariate Cox analyses (Table 3). Furthermore, and likely mediated by guideline-based management, LV volumes by standard imaging were significantly predictive of outcomes. LVEF, end-systolic wall stress, and global longitudinal strain had no significant association with outcome.
MEE remained a significant independent predictor when adjusted for any of the standard imaging parameters—more pronounced when a cutoff MEE of less than 25.7% was used. Table 4 shows the results of 2 experimental Cox multivariate models, in which an MEE of less than 25.7% was adjusted for the parameters with the highest univariate predictive capacity from either echocardiography or CMR. In a Cox model of MEE corrected for anamnestic presence of hypertension, MEE remained highly predictive (P = 0.0002), whereas history of hypertension did not reach statistical significance (P = 0.9).
Experimental Multivariate Cox Proportional-Hazards Models of MEE with Adjustments for Standard Outcome Parameters from Either Echocardiography or CMR
DISCUSSION
Our study shows for the first time, to our knowledge, the relation of MEE to mitral regurgitation severity, LV remodeling, and progression requiring surgical intervention in asymptomatic severe primary mitral regurgitation. Importantly, the predictive value of MEE was proportional and persisted after correction for standard objective estimators of mitral regurgitation severity, suggesting that MEE offers information that is orthogonal to the estimators recommended in current guidelines. These observations suggest that reduction of myocardial efficiency precedes progression to symptomatic mitral regurgitation requiring intervention.
MEE predicted outcome independently of standard clinical, laboratory, echocardiographic, and CMR parameters collected at the time of PET. This result can be partially explained by the fact that MEE is calculated from parameters that are typically not part of standard guideline-oriented mitral regurgitation evaluation, such as cardiac forward work and myocardial oxygen consumption. However, none of the functional parameters used in the MEE equation were significant predictors of outcome on their own.
Clinically, our data confirm the prognostic impact of well-established regurgitation parameters such as mitral regurgitant volume and LV volumes for both echocardiography and CMR. LVEF did not correlate with MEE in this cohort and was not predictive, probably because LVEF was within the reference range in all subjects.
Maximum tricuspid jet velocity and left atrial volume, the echocardiographic estimates of backward volume loading recommended in guidelines, were both significantly correlated with MEE and significant predictors in univariate analyses. Both, however, lost predictive significance when adjusted for MEE in multivariate models. A potential explanation for these results is that a poorer metabolic efficiency might have a culprit role in reducing diastolic LV function, which drives the backward failure and results in earlier symptom occurrence.
The experimental multivariate Cox analyses showed that end-systolic volume from echocardiography, but not from CMR, had independent predictive capacity. This is confusing, since CMR is the gold standard. A potential explanation could be that treating physicians had access to serial echocardiography data according to guidelines but were masked to CMR and PET.
Although MEE showed independent predictive value for outcomes, the modest size of our cohort, as well as the limited availability of 11C-acetate PET, does not allow us to predict the potential future role of MEE in the routine management of patients with asymptomatic severe mitral regurgitation. Still, the orthogonal perspective on progression offered by MEE might be useful for research into optimizing decision-making algorithms based on clinical data and for developing surrogate markers of MEE from standard imaging modalities. One such opportunity could be to study MEE in mitral regurgitation patients with concomitant cardiovascular or metabolic diseases to see if comorbidities contribute to mitral regurgitation progression by augmenting disturbances in oxidative metabolism beyond what is caused by volume overload. Hypertension is common in mitral regurgitation, and patients who have both disease entities are potentially prone to more rapid symptomatic progression, but there is no clear evidence for benefit of antihypertensive therapy (16). The fact that MEE is lowered in hypertension with LV hypertrophy (17) suggests that a history of hypertension might predispose a reduction of MEE in mitral regurgitation and contribute to the more rapid symptomatic progression in a subset of patients found in this study. On the basis of this hypothesis, we tested the association of hypertension and MEE for predicting progression but found no significant interaction in this cohort.
In patients with overt heart failure, MEE is significantly associated with LV hypertrophy and end-systolic wall stress (6). Among the individual parameters used in the MEE equation, LV mass was the only one that was significantly increased in mitral regurgitation patients, compared with healthy controls in our study. The hypertrophy seen in mitral regurgitation is generally regarded as an adaptive mechanism that reduces wall stress, secondary to LV dilatation. We did not find any association of end-systolic wall stress toward MEE or outcome, probably because the hypertrophic adaptation matched the dilatation sufficiently overall. However, this adaptation is apparently not sustainable in a subset of mitral regurgitation patients and causes a lowered metabolic efficiency even before major adverse changes in wall stress and systolic function occur. This may relate to metabolic alterations found in failing myocardium, including in the setting of mitral regurgitation (18), and points to a poorly understood variation in phenotypic susceptibility. MEE was in the reference range in mitral regurgitation subjects that did not experience early progression despite LV hypertrophy, suggesting that early adaptations to chronic volume overloading in subjects without increased susceptibility include a potentially improved efficiency of oxidative metabolism. This is analogous to previous findings in subjects with LV hypertrophy due to aortic stenosis and pressure overload, where MEE was in the reference range until symptom occurrence (19).
11C-acetate PET has been used to study MEE at a later stage in mitral regurgitation progression in small studies of symptomatic primary (7) and secondary (9) mitral regurgitation, showing MEE improvements after surgery in parallel with normalization of forward stroke volume. In the current study, external cardiac work and forward stroke volume in patients who reached the endpoint was not significantly different from that in patients who were censored or healthy volunteers, and symptom burden was minimal, suggesting that our cohort was studied at an earlier disease state than in previous 11C-acetate PET studies on mitral regurgitation. Moreover, it is difficult to draw conclusions from comparisons between our study and previous knowledge because guideline criteria for recommending surgical intervention have become more aggressive in the recent decade. Hence, studies with serial PET measurements might be required to understand the dynamics of myocardial metabolic efficiency, during progression leading to an intervention and during recovery after surgery, and to what extent the presumably distinct predictive value of MEE found in this study can be used for therapeutic decision making or as an outcome surrogate in drug trials. For such studies, PET-MEE has the advantage of simultaneous acquisition and automated analysis of all required parameters. The data show, however, that combined CMR and PET with careful avoidance of hemodynamic alterations between scans is not inferior to PET alone for predictive purposes, but MEE quantification appear to be method-dependent.
Several limitations of our study should be recognized. The number of patients included was modest, but nevertheless this was potentially the largest study evaluating primary mitral regurgitation severity with same-day PET, echocardiography, and CMR. Most importantly, this was a study on patients with moderate-to-severe or severe primary mitral regurgitation, which does not address lesser degrees of mitral regurgitation or secondary mitral regurgitation.
An important fundamental limitation was the nature of our endpoint, which was mitral valve intervention. The decision to proceed with intervention followed current guidelines at the time of the study and thus was triggered by the emergence of symptoms or N-terminal pro–brain natriuretic peptide increase, progressive LV dilatation, reduction in LVEF exceeding guideline-specified echocardiographic limits, or some combination of these features. Hence, it is not surprising that volumetric indices such as mitral regurgitant volume and LV end-diastolic volume were prognostic of outcomes. One patient was recommended for surgery but declined and died of cardiovascular causes. Of interest, this patient had the lowest MEE (15.7%) of all subjects in the cohort. Removal of this patient from outcome analyses did not change results or conclusions.
We acknowledge that MEE is a simplified approach to measuring myocardial efficiency and that comparisons to invasive approaches with pressure–volume loop analyses and direct measurement of mean myocardial oxygen uptake are relevant for future studies. Pump work did not include the product of regurgitant volume and end-systolic left atrial pressure; it is unclear whether this addition would alter the predictive value of MEE.
CONCLUSION
MEE by 11C-acetate PET was reduced in asymptomatic chronic degenerative mitral valve in proportion to the severity of mitral regurgitation measured by multiparametric echocardiography and CMR, and MEE predicted time to progression triggering surgical intervention, independently of standard imaging parameters of mitral regurgitation severity, LV function, and size.
DISCLOSURE
This study was supported by research grants 20130631 and 20190593 from the Swedish Heart-Lung Foundation, Stockholm, Sweden. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the role of MEE measured with 11C-acetate PET in progression of asymptomatic moderate-to-severe or severe primary mitral valve insufficiency?
PERTINENT FINDINGS: MEE was proportional to standard imaging indicators of regurgitation severity and volume overload. MEE was independently predictive of time to progression requiring surgical intervention.
IMPLICATIONS FOR PATIENT CARE: MEE might provide an objective and early indication of deteriorating myocardial energetics in mitral valve disease.
Footnotes
Published online Jan. 5, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 20, 2022.
- Revision received November 17, 2022.