Abstract
4055
Introduction: The PSMA-targeted radiation-based therapeutic PSMA-617 has been validated as a treatment for metastatic castration-resistant prostate cancer (mCRPC). Results from the VISION phase 3 clinical trial showed that 177Lu-PSMA-617 radioligand therapy (RLT) led to reductions in serum prostate specific antigen levels in 71.5% of patients. While PSMA-RLT is successful in mCRPC, alternative treatments for PSMA-negative prostate cancer like neuroendocrine prostate cancer are lacking. A promising alternative are small molecule radioligands targeting a serine protease called fibroblast-activation protein (FAP). FAP can be overexpressed on cancer-associated fibroblasts (CAFs, >90% of tumor stroma) as well as on certain cancer cells and expression is associated with tumor growth, neo-angiogenesis, metastasis formation, treatment resistance and immunosuppression. Recently, Kesch et al. highlighted the relevance of FAP expression in prostate cancer patients showing a significant rise in FAP expression with advancement of disease and high tumor accumulation in men with advanced CRPC. To compare the potential of novel FAP-targeted radioligand therapy (RLT) with PSMA-targeted RLT and to monitor alterations in target expression post RLT, we tested both RLTs in a PSMA+/FAP+ mouse tumor model with genetically engineered PSMA-positive PC3 cells (PC3-PIP).
Methods: Male NCG mice were inoculated with s.c. 5 x 106 PC3-PIP cells in 100 µl PBS/Matrigel (1:1). After 19 days, FAP expression was quantified in vivo by PET/CT 1h after i.v. administration of 1.1 MBq of 68Ga-FAPi-46. On day 24, tumor volumes were determined by CT and animals were randomized into three groups (n=19/group; control, FAP-RLT, PSMA-RLT). On day 27 p.i. (average tumor volume: 100 mm3), the RLT groups received either 30 kBq 225Ac-PSMA-617 or 40 kBq 225Ac-FAPi-46. For efficacy testing, tumor volumes (n=11/group) were subsequently monitored by weekly CTs and post-treatment PSMA and FAP expression was monitored by PET/CT scans (n=4/group) with 68Ga-FAPi-46 (after 1 week) and with 68Ga-PSMA-11 (after 2 weeks). Additional tumors were collected and paraffin-embedded at day 1 and 21 after RLT (n=4+4/group) for subsequent IHC staining of human PSMA and murine FAP. Therapeutic efficacy was assessed by tumor volumetry (CT), time to progression (TTP) and survival. For IHC on clinical samples, 210 tissue microarray (TMA) cores of CRPC (70 tumors), including locally advanced CRPC (N=14) from John Hopkins Hospital and mCRPC (N=56) from the University of Washington (Autopsy TMA program), were classified by androgen receptor (AR) and neuroendocrine (NE) status and stained for PSMA and FAP expression. OsiriX Software was used for PET/CT analysis and CT volumetry. GraphPad Prism was used for statistical analysis.
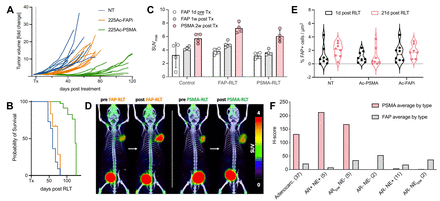
Results: Compared to untreated controls (60d), FAP-RLT showed efficient tumor growth delay resulting in significantly prolonged median survival of 74d (p=0.044) while median survival for PSMA-RLT reached 123d (p≤0.0001), recapitulating clinical findings from the VISION trial. (Fig.1A/B) Pre-treatment 68Ga-FAPi-46 PET/CT scans revealed average SUVmax of 3.4±1.1. Post-treatment PET/CT scans showed significantly higher SUVmax for FAP-RLT (p=0.028) and non-significant increases in SUVmax for control and PSMA-RLT. (Fig.1C/D) IHC stainings showed a decrease of FAP+ cells per μm2 from day 1 to day 21 post PSMA-RLT, while minor increases were observed for control and FAP-RLT. (Fig.1E) Clinical PSMA expression was high in AR-positive (H‑score= 132-213) and low in AR-negative lesions (H‑score = 1‑4). FAP expression was irrespective of AR status, but was higher in NE-negative (H-score= 22-53) compared to NE-positive prostate cancer (H-score= 8-18). (Fig.1F)
Conclusions: Both 225Ac-FAPi-46 or 225Ac-PSMA-617 induced significant tumor growth delay in the PC3-PIP model. Our preclinical data together with clinical FAP expression in all subtypes of CRPC support the role of FAP-RLT for the treatment of advanced PSMA-negative prostate cancer.