Abstract
3332
Introduction: Myocardial ischemia-reperfusion (I/R) injury is associated with systemic oxidative stress, cardiac mitochondrial homeostasis, and cardiomyocyte apoptosis. Metformin was recognized to attenuate cardiomyocyte apoptosis. However, the longitudinal effects and pathomechanism of metformin on the regulation of myocardial mitohormesis following I/R treatment were unclear. This study aimed to investigate the longitudinal effects and mechanism of metformin on regulating cardiac mitochondrial homeostasis by serial imaging with a 18-kDa translocator protein (TSPO) targeted positron emission tomography (PET) tracer 18F-FDPA.
Methods: Myocardial I/R injury was established in SD rats, which were treated with or without metformin (50 mg/kg per day). Serial gated 18F-FDG and 18F-FDPA PET imaging were performed at 1, 4, and 8 weeks after surgery, ventricular remodeling and cardiac mitochondrial homeostasis were analyzed, respectively. After PET imaging studies, the activity of antioxidant enzymes, immunostaining and western blot analysis were performed to analyze the spatio-temporal effects and pathomechanism of metformin for cardiac protection after myocardial I/R injury.
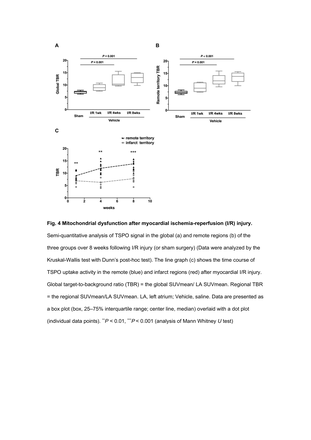
Results: Oxidative stress and apoptosis increased one week after myocardial I/R injury (before significant progression of ventricular remodeling), TSPO co-localized with inflammatory CD68+ macrophages in the infarct area, and upregulation of AMPK-p/AMPK and down-regulation of Bcl-2/Bax were observed. However, these effects were reversed with metformin treatment. Eight weeks after myocardial I/R injury (advanced stage of heart failure), 18F-FDPA uptake activity in myocardial cells in the distal non-infarct area increased without CD68+expression, whereas it was decreased with metformin treatment.
Conclusions: Prolonged metformin treatment has pleiotropic protective effects against myocardial I/R injury associated with a regional and temporal dynamic balance between mitochondrial homeostasis and cardiac outcome, which were assessed by TSPO-targeted imaging during cardiac remodeling.