Abstract
2996
Introduction: Approximately 10-22% of metastatic neuroendocrine tumors (NETs) can be unknown primary (CUP). The application of somatostatin receptor (SSTR)-PET provides a remarkable improvement in primary tumor detection, however, in a number of cases no primary tumor is found even with SSTR-PET and after prolonged follow-up. Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogues has been shown to be highly efficacious for the systemic treatment of many types of metastasized well/moderately differentiated NETs with a known primary site, include gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Up to now, limited data are available concerning PRRT in patients with neuroendocrine tumors of unknown primary (CUP-NETs). The purpose of this study was to analyze the long-term outcome, efficacy and safety of PRRT in patients with CUP-NETs after the introduction of the SSTR-PET.
Methods: A total of 149 patients with pathologically confirmed metastatic CUP-NETs were treated with lutetium-177 (177Lu) and/or yttium-90 (90Y) labeled somatostatin analogs (DOTATATE or DOTATOC) between June 2003 and March 2019. All patients underwent 68Ga-SSTR PET/CT and 133 patients underwent 18F-FDG PET/CT before PRRT. Treatment response, according to RECIST 1.1 (CT and/or MRI), and by molecular imaging criteria (PERCIST) were assessed. Kaplan-Meier analysis was performed to calculate progression-free survival (PFS) and overall survival (OS), defined from start of PRRT. Short- and long-term adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
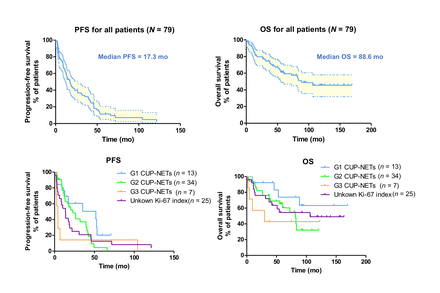
Results: 68Ga-SSTR PET/CT detected the primary tumor in 70/149 (47%) of the patients, whereas primary tumor was positive on 18F-FDG PET/CT in 17/133 (13%). 79 patients (41 males, 38 females; age 28-85 y, mean age 60.0 ± 13.0 y) remaining CUP-NETs after 68Ga-SSTR PET/CT and other examinations were reviewed in the study. Among them, 61 patients (77.2%) had liver metastases, 41 (51.9%) had lymph node metastases, 6 (7.6%) had lung metastases, 30 (38.0%) had bone metastases, and 19 (24.1%) had metastasis of other organs. 13 (16.5%) had G 1 NETs, 34 (43.0%) had G2 NETs, 7 (8.9%) had G3 NETs and 25 (31.6%) were with unknown Ki-67 index. Thirty-one (39.2%) patients received dual PRRT with the combination of 177Lu and 90Y; 32 (40.5%) received 177Lu as monotherapy, and 16 (20.3%) received 90Y as monotherapy. The mean cumulative administered radioactivity for all patients was 18.8 ± 11.4 GBq (range, 2.5 - 54.6 GBq). The median follow-up time was 92.3 mo, with the shortest duration of 4.0 mo and the longest of 169.1 mo. The median PFS was 17.3 mo and the median OS was 88.6 mo. For G1 CUP-NETs (n=13), G2 CUP-NETs (n=34), G3 CUP-NETs (n=7), and unknown Ki-67 index CUP-NETs (n=25), the median PFS were 51.9 mo, 22.9 mo, 4 mo, 13.7 mo and median OS were not yet reached, 81.6 mo, 29.9 mo, 106.3 mo, respectively. PRRT was well tolerated by all patients. During the treatment and long-term follow up, CTCAE grade 3 anemia and leukocytopenia occurred in one (1.3%) and another one patient (1.3%), respectively. No grade 3 thrombocytopenia was observed. No CTC-4 anemia, leukopenia, or thrombocytopenia was observed after treatment. No grade 3 or 4 nephrotoxicity or any clinically significant decline in renal function was observed. There was no hepatic toxicity.
Conclusions: PRRT is a favorable therapeutic option in patients with metastatic CUP-NETs that express somatostatin receptors, and tolerated well with few side-effects. PRRT has shown good response rates and results in encouraging long-term outcome in this large cohort of patients with CUP-NETs with a follow up of up to 14 years after the commencement of both SSTR-PET and PRRT. Further prospective studies are warranted.