Abstract
2965
Introduction: Alzheimer’s disease (AD) is a neurodegenerative disease characterized by aggregation of Aβ plaques and Tau protein causing senile plaques (SP) and neurofibrillary tangles (NFT) in the brain. Imaging of SP and NFT using PET has confirmed initial accumulation in AD hippocampal regions which progressively spreads to cortical regions and may be related to Braak stages and clinical diagnosis of AD. We have developed a Tau selective radioligand, [125I]IPPI suitable for autoradiographic studies of NFT and [18F]flotaza suitable for autoradiographic studies of SP in AD brains. Because there may be regional differences in the location and load of NFT and SP in the AD brain specimens, we have compared the binding of [125I]IPPI and [18F]flotaza in HP (CA1/subiculum) in postmortem AD brain slices (n=29) and compared to cognitively normal (CN, n=32) brains.
Methods: Hippocampus (CA1/subiculum plus) of AD (n=29; 13 male and 16 female) and control subjects (n=32; 16 male and 16 female) from well-characterized subjects were used in the study. All brain sections (10 μm thick, freshly frozen) were obtained from Banner Health Research Institute, Sun City, Arizona. In vitro autoradiography studies were carried out with [125I]IPPI for Tau and [18F]flotaza for Aβ in AD and CN brains. Brain slices with [125I]IPPI and [18F]flotaza in ethanolic PBS buffer pH 7.4 (0.1 μCi/mL and 1 μCi/cc, respectively) were incubated at 25 oC for 1.25 hr. Nonspecific binding was measured in the presence of 10 μM MK-6240 and 10 μM PIB. The slices were then washed with cold PBS buffer, 50% ethanolic PBS buffer twice, PBS buffer and cold water. The brain sections were air dried, exposed overnight on a phosphor film, and then placed on the Phosphor Autoradiographic Imaging System/ Cyclone Storage Phosphor System and extent of binding of [125I]IPPI and [18F]flotaza were measured in DLU/mm2. Adjacent slices were immunostained (IHC) with anti-Tau for total Tau and anti-Aβ for plaques using reported protocols. QuPath was used for analysis of the IHC images.
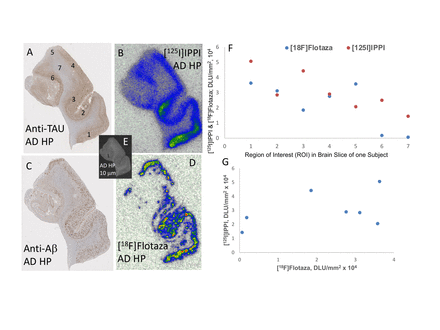
Results: All AD subjects exhibited high [125I]IPPI (Fig-1B) and [18F]flotaza (Fig-1D) binding in the HP CA1-SUB grey matter (GM) regions. The binding of both the radiotracers corresponded to their IHC stains (Fig-1A,C). The CN subjects had significantly lower binding as expected for [125I]IPPI while it was non-existent for [18F]flotaza, unless there were small amounts Aβ plaques present in the CN brain samples(confirmed by IHC). Ratio of average GM [125I]IPPI in AD versus white matter (WM) was found to be >3, whereas for [18F]flotaza GM/WM >50. Within a given subjects HP, there were regions of low, medium and high binding for both [125I]IPPI and [18F]flotaza. Figure-1 shows comparison of binding in HP of one subject in seven different regions. As can be seen in Fig-1B and D, there were regions of overlap as well as regional differences. This resulted in a lack of linear correlation (Fig-1G) between [125I]IPPI and [18F]flotaza. A more detailed correlative analysis of [125I]IPPI and [18F]flotaza with IHC intensity is currently being carried out using QuPath. This will allow a better understanding of potential variations in the distribution of SP and NFT within the brains of each AD subject. Additionally, white matter regions as reference regions within each subject may provide more accurate inter-subject comparisons. This additional analysis is currently ongoing.
Conclusions: [18F]Flotaza and [125I]IPPI are excellent probes for studying SP and NFT in the human postmortem AD brains. Our studies indicate that there is a mismatch in the location of SP and NFT and therefore their effects on other neuronal elements in different brain areas may be either additive or singular.