Abstract
2876
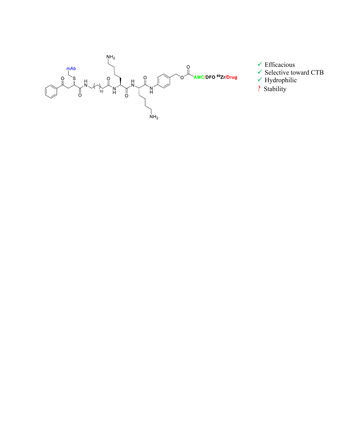
Introduction: Antibody drug conjugates (ADCs) have emerged as a promising cancer treatment due to their high specificity and affinity to recognize cancer-associated receptors, delivering the drug into the tumour. Although eleven ADCs have been U.S Food and Drug Administration (FDA) approved, there are currently more than 80 ongoing clinical trials. The linker between the antibody and the drug in ADCs plays a key role in the effectiveness of the ADC. Among different types of linkers, enzymatic cleavable linkers are more common, considering six of the FDA approved ADCs. They are designed to release a highly potent cytotoxic drug in the tumor environment by tumour associated protease enzymes like Cathepsin B (CTB). Although increased catalytic activity of CTB is found in aggressive and metastatic cancers, no systematic studies evaluating how changes in the linker structure affects the rates of drug release by CTB. Instead, Val-Cit dipeptide is the most CTB cleavable linker used for ADCs. However, this recognition sequence can be cleaved by other proteases and presents poor serum serum stability in mice leading to poor treatment efficacy in preclinical studies. Thus, efficacy, rodent plasma instability, and high hydrophobicity are some of the known linker drawbacks for ADCs that employ Val-Cit, which could lead to side effects and inefficacy in humans. This suggests that linkers with higher rates of hydrolysis coupled with higher specificity towards CTB may be beneficial in the design of ADCs.
Methods: Four LysLys containing dipeptide linkers were designed, synthesized, and evaluated as novel linker conjugate models bearing a 7-amino-4-methylcoumarin (AMC) fluorophore reporter instead of a drug. The effects of linker structure on protease cleavage rates and selectivity were measured by fluorescence using human recombinant CTB to determine kinetic parameters (kcat, KM, kcat/KM) followed up by relative rates of hydrolysis in lysates prepared from MDA-MB231, DU-145, and SKBR3 cancer cells. Serum stability was evaluated by incubation of the various linkers or linkers incorporated into peptide conjugates with human or mouse serum for different times to determine percentage of degradation. Currently, we are developing [89Zr]Zr-labeled radioimmunoconjugates conjugated to anti-MUC1 mAb AR20.5 (Quest Pharmatech) using desferrioxamine (DFO) as a chelator conjugated to the antibody using three different dipeptide linkers; LysLys, ValCit and a non-cleavable linker. The radioimmunoconjugates allows us to mimic ADC behavior in vivo and determine linker stability and biodistribution in mice bearing MUC1-expressing SKOV3 xenograft models using positron emission tomography (PET) imaging.
Results: The four LysLys bearing linkers showed higher rates of hydrolysis and selectivity towards CTB compared to ValCit, the most common cleavable linker used in ADC design. In vitro studies showed that each unconjugated linker molecule was not stable in human and mouse serum, however serum stability was dramatically improved after conjugation with larger molecules such as peptides or proteins. Once synthesized the [89Zr]Zr-labeled radioimmunoconjugates will be studied in vitro and in vivo to determine the linker stability and biodistribution in SKOV3 xenograft models.
Conclusions: The chemical synthesis of several LysLys-based linkers coupled to AMC was established in good yields. In vitro studies of the LysLys-based linkers using AMC to report on protease hydrolysis support their use as alternative dipeptide linkers for CTB cleavable ADCs due to faster rates of hydrolysis and high selectivity compared to the “gold standard” ValCit.