Abstract
2852
Introduction: Nicotinic acetylcholine receptors (nAChRs) α4β2* subtype play an important role in the CNS and have been implicated in neurodegeneration. In Alzheimer’s disease (AD), aggregation of b-amyloid proteins forms plaques that potentially interact with neuronal receptors and disrupt cellular function. Our preliminary work using [18F]Nifene, a selective fast-acting α4β2* nAChRs PET radiotracer, on postmortem human AD brain slices suggests a potential loss of α4β2* nAChRs in AD. To study the effects of b-amyloid plaques in vivo, we have now used the transgenic 5xFAD AD mouse model which overexpresses the mutant human amyloid precursor protein with 5 Familial Alzheimer’s Disease (5xFAD) mutations. These mice develop amyloid pathologies quickly, causing inflammation, microgliosis, synaptic and neuronal loss. 5xFAD mice >12 mo old may be a good representation of the b-amyloid plaques in human AD. Here we report our preliminary findings on [18F]Nifene PET/CT in the 5xFAD AD mouse model.
Methods: Male and female hemizygous 5xFAD (n=10) and no-carrier male and female (n=4) 12-16-month old mice from Jackson Labs were used in the study. All mice were fasted for at least 24 hours. [18F]Fluoride (from PETNET solutions) was used to prepare [18F]Nifene, which was injected intraperitoneally (IP) in normal saline (approx. 2 MBq in 0.1 mL) under 2% isoflurane anesthesia. They were placed in the supine position in a mouse holder and anesthetized with 2% isoflurane for whole-body PET/CT imaging. All mice underwent a 15 minute-long PET scans acquired 40 minutes after [18F]Nifene injections. All animals had a 7-minute-long CT scan before the PET scan for attenuation correction and anatomical delineation of PET images. An Inveon Multimodality (MM) CT scanner (Siemens Medical Solutions, Knoxville, TN) was used for PET/CT acquisitions. Select 5xFAD mice brains used in the study were sectioned and presence of Ab plaques was confirmed by [18F]flotaza autoradiography.
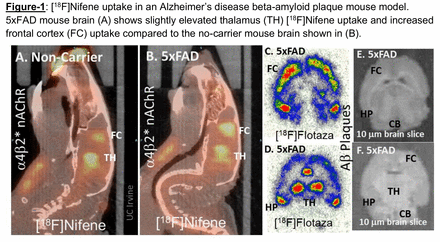
Results: The magnitude of [18F]Nifene uptake in the brain was expressed as standard uptake value (SUV) which was computed as the average [18F]Nifene activity in each volume of interest, VOI (in kBq/mL) divided by the injected dose (in MBq) times the bodyweight (Kg) of each animal. [18F]Nifene binding in the no-carrier mouse was as expected, with the highest levels in the thalamus (TH) followed by frontal cortex (FC) and other brain regions (Fig-1A). Uptake levels within the 5xFAD mice were varied, with 4 of the mice exhibiting a similar uptake pattern compared to the no-carrier mice. Three 5xFAD mice showed an increase in overall frontal cortex uptake and had equivalent values for the thalamus and frontal cortex (Fig-1B). Three 5xFAD mice showed higher levels of [18F]Nifene within the frontal cortex. Average SUV values 5xFAD: Group 1: TH: 2.38±0.49 FC: 1.92±0.33, Group 2: TH: 2.86±0.68 FC: 2.11±0.46, Group 3: TH: 2.79±0.58 FC: 2.81±0.42, Group 4: TH: 2.37±0.65 FC: 3.28±0.42 (Fig-2). Autoradiography of 5xFAD mice brains using the Ab plaque radiotracer [18F]flotaza shows extensive Ab plaques present in FC, TH and hippocampus (HP) (Fig-1C,D). Ratio of [18F]flotaza in these brain regions versus cerebellum (CB) was >50. The binding of [18F]Nifene in the control mice and 5xFAD mice was displaced by nicotine injected IP in the brain regions. However, the clearance rates in FC appeared to be slower in FC compared to TH in 5xFAD mice. Further studies are underway to establish the nature of bound [18F]Nifene in in 5xFAD mice.
Conclusions: Significantly increased [18F]Nifene uptake in the 5xFAD AD mouse model was observed in the frontal cortex at 12-16 months of age. Since these mice are affected by inflammation, microgliosis, synaptic, neuronal loss and other motoric dysfunctions, it remains to be determined which of these pathologies may be contributing to the increased [18F]Nifene binding. Additionally, the cortical increase in [18F]Nifene binding could be due to learning and memory deficits inflicted by Ab plaque accumulation.