Abstract
2723
Introduction: Artificial intelligence (AI) algorithms are being incorporated into modern healthcare, and they are increasingly applied in radiology and molecular imaging. However, opportunities to integrate AI for pediatric imaging have received surprisingly little attention thus far. Integrated PET/MRI has shown significant clinical value for staging and restaging of children with cancer by providing functional and anatomic tumor evaluation with a 1-stop imaging test and with up to 80% reduced radiation exposure compared with 18F-FDG PET/CT. The goal of this educational exhibit is to share our experience and review the current utilization of PET and MRI imaging-based AI models in pediatric oncology.
Methods: We conducted a literature search in PubMed, and Web of Science to identify the use of AI-based approaches for pediatric oncology imaging applications: search terms included Artificial Intelligence, Machine Learning, AND children or pediatrics or young adults AND PET/MRI or PET or MRI AND cancer. We determined the proportion of AI applications for pediatric cancer imaging that were applied for the above-mentioned criteria and present an overview of potential applications and their clinical impact.
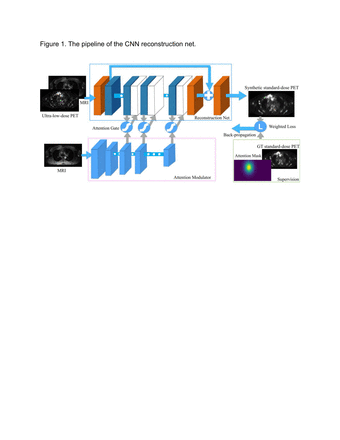
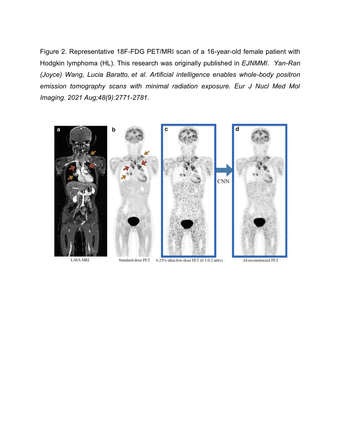
Results: We will present examples of AI-based image data processing algorithms for children with cancer, which have been utilized in our own practice. We will also show results from our literature search which revealed 10 articles on PET and MRI imaging-based AI algorithms for pediatric cancer, all published in the last 3 years. Our group published 2 articles on the use of AI to reduce radiation exposure of pediatric oncology imaging studies. We implemented Convolutional Neural Networks (CNNs) to combine inputs from simulated low-dose 18F-FDG PET scans and simultaneously acquired MRI scans to produce a standard dose 18F-FDG PET scan, which provided equal diagnostic accuracy and quantitative SUV measurements compared to clinical standard 18F-FDG PET images (Figure 1 and 2).
MRI has been used to implement AI algorithms for the detection and classification of pediatric brain tumors. For example, T2-weighted MRIs inputs were used in 617 children with posterior tumors and 199 controls to implement a 2D ResNeXt-50-32x4d deep learning architecture able to detect the presence of a tumor and predict tumor class. Results from this multicenter study showed comparable tumor-detection and classification accuracy between the model and 4 board-certified radiologists.
Diffusion-weighted Imaging (DWI MRI) and Apparent Diffusion Coefficient (ADC) have been studied to implement AI algorithms for segmentation purposes on children with osteosarcoma. Our group is now developing a PET/MRI AI model for the classification of treatment response in pediatric lymphoma patients.
Other possible applications of AI in pediatric oncology imaging would be for reducing the scanning time, for test selection, for image quality control, for image triage, and for image reporting. While initial applications were conducted in adult patients, these algorithms would be of potential high relevance for pediatric cancer patients as well.
Conclusions: We shared our experience and reviewed the current utilization of AI for imaging of children with cancer. PET/MRI-based AI models have been implemented mostly for the reduction of radiation exposure, while solely MRI-based AI algorithms have been used for detection, segmentation, and classification purposes. Future directions would explore AI software systems to accelerate scan times, streamline workflows and image reporting.