Abstract
2583
Introduction: Dry mouth (xerostomia) is one of the most common complications suffered from head and neck (H&N) cancer patients, which can impair the patient’s ability to swallow and lead to long-term oral disorders. Additionally, radiation-induced xerostomia may result from radiopharmaceutical therapy (RPT), in which excess radiation dose is imparted to the salivary glands (SGs). Due to the low effectiveness of xerostomia management strategies, there is significant motivation to reduce SG toxicity resulting from EBRT and RPT. One possible strategy to minimize risk is to improve delineation of SGs via functional imaging. Recent developments of positron emission tomography (PET) targeting prostate specific membrane antigen (PSMA) has allowed for the observation of SGs with high image contrast. In this study, we evaluate the feasibility of enhancing SG delineation with PSMA PET imaging using radioactive SG models created with Negative Cast Modelling (NCM). We focus on optimizing PET segmentation to match the true organ volume, although these methods may be modified based on the clinical objective, such as to account for error margins during treatment planning.
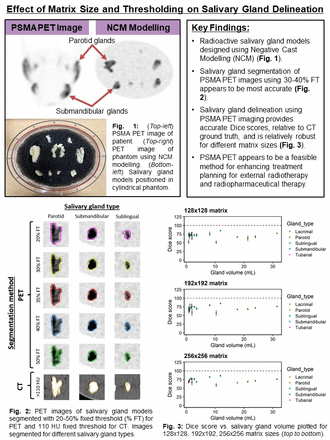
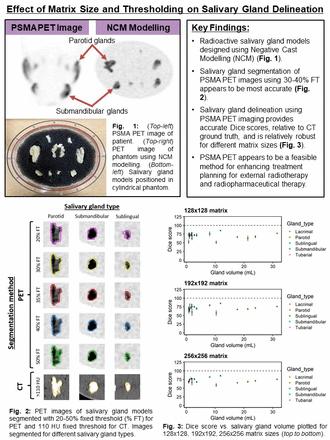
Methods: Salivary gland models were cast using NCM based on segmented templates from 4 patients imaged with [18F]DCFPyL PET. 18F concentration was based on an analysis of 360 salivary glands from 40 patient images. The salivary gland models were positioned in a cylindrical water phantom with polyurethane filter foam, and scanned twice using a GE D690 PET/CT scanner. The first scan was used to determine the ground truth concentration of 18F in the SGs (10min bed duration). Next, the phantom background was injected with [18F]FDG which corresponded to the patient analysis (2.5 kBq/mL). Five 2min frames were acquired, and images were reconstructed using Bayesian sequential regularized expectation maximization (BSREM) algorithm (32 subsets, 25 iterations, <m:omath>γ=2</m:omath>, β=300) using 128x128, 192x192, and 256x256 matrix sizes. SGs were segmented using 20-50% fixed threshold (FT). Computed tomography (CT) images were used to provide the ground truth segmentation, by thresholding voxels >110 HU. Dice coefficients were computed for each salivary gland. Mean dice score (MDS) and mean percent noise (MPN) were computed as measures of accuracy and precision, respectively. Gland deviation (GD), defined as the standard deviation of dice scores between different types of SGs, was used to evaluate the robustness of each segmentation method.
Results: To select the most accurate and robust segmentation threshold, MDS<m:omath>±</m:omath>GD was compared for the 192x192 matrix size. MDS<m:omath>±</m:omath>GD were 67.5<m:omath>±</m:omath>14.9, 72.9<m:omath>±</m:omath>10.1, 74.6<m:omath>±</m:omath>7.0, 73.6<m:omath>±</m:omath>6.6, 71.1<m:omath>±</m:omath>7.3, 61.5<m:omath>±</m:omath>9.9%, for the 20, 25, 30, 35, 40, 50% FTs, respectively. Percent noise vs. bias curves were plotted for the 35% FT with different matrix sizes. MDS<m:omath>±</m:omath>GD were 71.8<m:omath>±</m:omath>8.7, 73.6<m:omath>±</m:omath>6.6, 75.0<m:omath>±</m:omath>8.5%, and MPN were 3.5, 2.9, 2.2%, for the 128x128, 192x192, and 256x256 matrix sizes. To compare segmentation for different types of salivary glands, percent noise vs. bias curves were plotted using the 35% FT with 256x256 matrix size. MDS<m:omath>±</m:omath>PN was 81.2<m:omath>±</m:omath>1.2%, 76.4<m:omath>±</m:omath>2.6%, 73.3<m:omath>±</m:omath>3.5%, 71.0<m:omath>±</m:omath>1.1%, and 66.6<m:omath>±</m:omath>3.7%, for the submandibular, sublingual, lacrimal, parotid, and tubarial glands, respectively.
Conclusions: Our results suggest using a 35% FT and 256x256 matrix size to achieve the most accurate and robust delineation of salivary glands in PSMA PET images. Using the BSREM algorithm (32 subsets, β=300) with 35% FT, we observed higher accuracy and reduced noise for smaller salivary glands (submandibular, lacrimal, sublingual) compared to larger glands (parotid and tubarial). In future studies, we will optimize SG segmentation for different types of salivary glands, scan durations, and reconstruction parameters. PSMA PET could potentially aid EBRT for treatment plans that require caution near the SGs. However, further studies are needed to investigate how this strategy may be translated to a clinical setting.